The coronavirus disease 2019 (COVID-19) pandemic started in Wuhan, Hubei Province, China, in December 2019, and by 24 April 2020, it had affected >2.73 million people in 185 countries and caused >192,000 deaths.1 The pathogen, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was believed to have reached humans through a zoonotic infection.2 SARS-CoV-2 is the third coronavirus outbreak in two decades, following the SARS-CoV outbreak in November 2002 in Foshan, Guangdong, China, which was subsequently contained, and the Middle East respiratory syndrome coronavirus (MERS-CoV) outbreak in 2012. All three have bats as the primary reservoir, but unlike SARS-CoV and MERS-CoV, where the civet cat and the camel have been detected as the intermediary hosts, respectively, this remains unknown for SARS-CoV-2.3 COVID-19 is characterised by flu-like symptoms, such as fever, cough and shortness of breath. Approximately 15–20% of COVID-19 cases evolve into severe diseases, such as pneumonia, that require hospital admission, and approximately 5% of patients develop critical conditions, such as acute respiratory distress syndrome, requiring ventilatory support, as well as multiorgan failure and death.4
Epidemiologically, these three coronaviruses share a similar incubation time (mean of ~5 days). MERS-CoV leads to death in one-third of cases and SARS-CoV in one-tenth; for SARS-CoV-2, reports indicate rates between 2% and 11% in confirmed cases.1,3 Notably, testing has been restricted to hospitalised patients in many territories. The basic reproduction number or new cases caused by one infected person characterises the virus spread (R0 = R nought).5 If R0 <1, the transmission is likely to stop. If R0 >1, there is a high risk of epidemic spread if there is not timely and effective containment. Although MERS-CoV has not been contained, it is currently controlled, due to R0 ~1. In contrast, SARS-CoV had an R0 ~4, and reached 29 countries and a total of 8,096 cases, with the last cases reported in May 2004.6 The reported R0 for SARS-CoV-2 is ~3; however, its current spread may indicate this to be higher.7,8 Importantly, the fact that infected individuals could become contagious before developing symptoms may augment R0. Despite diverse societal measures to avoid the spread of SARS-CoV-2, such as social distancing, self-isolation, quarantines, curfews and total lockdowns, which limit spread significantly, its control seems yet remote.
With the number of COVID-19 patients increasing exponentially, and a significant minority requiring hospitalisation and intensive care support,4 hospitals have massively reallocated resources to the pandemic, reduced elective care activities and limited the availability of investigators and research personnel to continue activities for ongoing clinical trials. Moreover, coordinating centres and sponsors in affected countries have shifted to home-based organisations, requiring adaption of operations to the current crisis. In addition, participants in clinical trials may not be able to attend hospitals for follow-up visits or to collect study medications. A careful and periodic risk assessment by sponsors and investigators is required to preserve the safety of trial participants (and employees) and the integrity of trials. In this article, we summarise the immediate implications of the COVID-19 pandemic on ongoing cardiovascular trials.
Regulatory Framework
This review incorporates recent recommendations from the US Food and Drug Administration (FDA), the European Medicines Agency, the UK’s Medicines and Healthcare products Regulatory Agency and Australia’s Therapeutic Goods Administration, as well as personal views.9–14
Basic Principles
Planning, executing and reporting clinical trials designed for the approval of (or to extend indications for) drugs, biological products, devices and combinations thereof, are highly regulated activities. Clinical trialists must observe national regulations, as well as international standards, such as those proposed by the International Conference of Harmonization, the International Organization for Standardization and the International Medical Device Regulators Forum.
Two general principles governing the execution of clinical trials are ensuring patient safety and clinical trial integrity. According to the WHO, patient safety is “the absence of preventable harm to a patient during the process of health care and reduction of risk of unnecessary harm associated with health care to an acceptable minimum.”15 As defined by the FDA, data integrity refers to “the completeness, consistency, and accuracy of data. Complete, consistent and accurate data should be attributable, legible, contemporaneously recorded, original or a true copy and accurate (ALCOA).”16 Data are to be recorded exactly as intended, and when retrieved at a later time, should be the same as originally recorded. While patient safety is paramount, both should be prioritised for the successful execution of clinical trials. If data integrity is compromised, study results may no longer be interpretable, reliable or usable.
Impact of a Pandemic on the Conduct of Clinical Trials
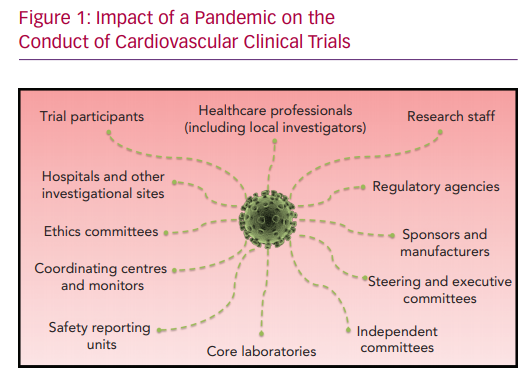
A pandemic has the potential to directly impact all individuals and organisations involved in clinical research (Figure 1). Highly contagious and rapidly spreading viruses, such as SARS-CoV-2, require comprehensive measures to avoid human-to-human spread. With the ongoing pandemic, the world has progressively witnessed a reduction of airline activity to almost zero with widespread travel bans, and limitation of private and public transportation, temporary closure of retail businesses, banning of public gatherings and the requirement to work from home. All these measures are designed to limit exposure to potential carriers of the virus. Individual measures, such as meticulous hand hygiene, self-isolation and social distancing, are encouraged. Public measures, such as quarantines, curfews or lockdowns, have been implemented. However, sectors, such as healthcare, food supply chains, law enforcement, governmental agencies and regulatory bodies, remain indispensable, with an increased workload challenging the capacity of local and national systems, as well as risking (if not sufficiently protected) the well-being of individuals. Overall, the majority of people stay at home, work remotely and limit use of healthcare systems as much as possible.
Impact on the Clinical Trial Life Cycle
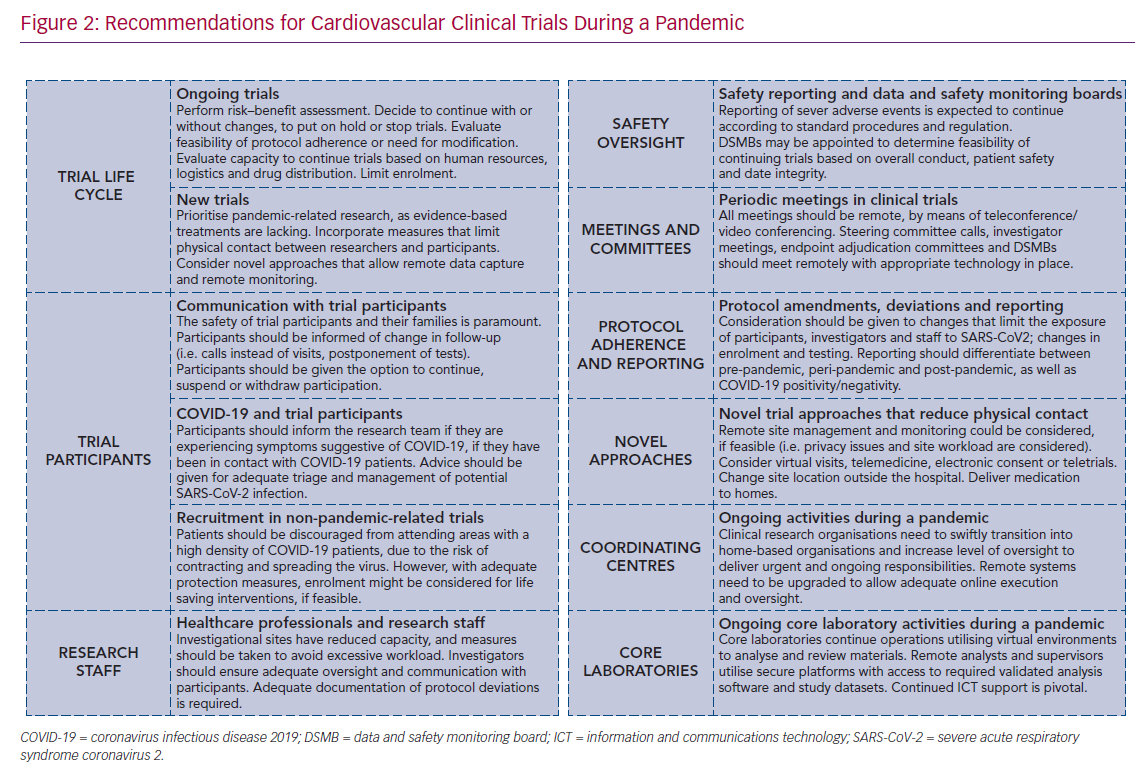
The clinical trial life cycle can be divided into trial design and registration, trial start-up, enrolment, follow-up, reporting and regulatory submission. For non-pandemic-related trials that have not yet started, feasibility should be critically assessed, and postponement strongly considered (Figure 2). For trials that are enrolling, activation of new sites should be postponed. Moreover, given that enrolment requires adequate assessment of eligibility, including protocol-required tests and written informed consent procedures, enrolment should be suspended or, if possible, slowed down. Follow-up visits should be performed via telehealth when possible. Extension of follow-up periods may be required to facilitate complete data collection. The reporting of adverse events should continue as usual.9–11,13,14 The reporting of trial results is encouraged through virtual international conferences. Overall, priority is given to pandemic-related clinical trials.
Changes in trial conduct should be documented and, if substantial, incorporated as protocol amendments (although not in an expedited manner unless impacting on patient safety); lesser changes may be captured as protocol deviations related to the pandemic. Regulatory agencies offer a diverse range of flexibility in such procedures, and applicable guidance documents should be consulted to establish the most appropriate approach for a particular trial.9–11,13,14
Trial enrolment should be put on hold or stopped if there is significantly reduced feasibility (e.g. drug trials with infusions), when participants require intensive care post-treatment (e.g. surgical trials) or when the investigator is unavailable. When inclusion is delayed by the pandemic, it should be dealt with in a similar manner to other circumstances that lead to a low recruitment rate. If appropriate, and especially if foreseen by the protocol, a data and safety monitoring board may assess futility due to severe impact on data collection or outcomes. However, if stopping or putting on hold a trial puts participants at increased risk, efforts should be taken to continue with trial-related activities.
Impact on Trial Participants
Enrolment in cardiovascular trials generally takes place at outpatient visits or during hospitalisation. Trials in patient populations with acute presentations (e.g. ST-elevation MI [STEMI]) may identify potentially suitable trial candidates; however, the capacity to comply with study procedures needs to be assessed, as well as considerations related to patient safety during follow-up. It is also pertinent to consider that COVID-19 may mimic some classical presentations, such as STEMI; ECG changes are shown to reflect myocarditis, after angiography demonstrates non-obstructive disease. It is problematic when the trial design mandates a protocol-related treatment before angiography. Furthermore, the analysis of outcomes may be rendered more difficult, and parallel analyses of the intention-to-treat and per protocol populations will be pertinent. Participants in the follow-up phase (when they are generally at home) constitute a higher-risk population in the COVID-19 pandemic. In addition, with isolation measures, participants have limited access to investigational sites. Therefore, a switch to telehealth (with data protection measures in place) should be considered, as well as postponement/cancellation of face-to-face visits. Ascertainment of primary endpoints should be prioritised, and if these need a particular test, consideration should be given to local laboratories or imaging centres closer to the participant’s home.
Postponing or changing follow-up or study intervals/windows, or not performing secondary assessments, may be considered. Importantly, a common approach to follow-up during the pandemic should be agreed upon among sponsors and investigators, and investigators should inform trial participants of any changes by means of newsletters or individual communications, including aspects affecting follow-up visits. Communication is critical. When adjusting visits or study procedures, the patient perspective must be captured. More specifically, participants may need to re-consent after being informed of the changes with the explicit possibility of stopping participation. Notwithstanding, participants should not visit investigational sites for the purpose of re-consenting. An alternative approach is a video call supplemented with a confirmatory email.11 Drug trials add the complexity of drug supply and the need to maintain blinding. Consideration should be given to shipping drugs directly to homes from the investigational sites, ensuring that transportation and storage conditions are appropriate. Drug accountability and compliance should be monitored.9,11,13,14
Impact on Healthcare Professionals and Research Staff
General medical practices, emergency departments and intensive care or pulmonary units are most exposed to direct contact with known COVID-19 patients. An excessive workload is also evident in imaging, laboratory and pharmacy departments, as well as other departments supporting the care of hospitalised COVID-19 patients, generally elderly people with accompanying comorbidities. Healthcare professionals from every discipline have been called upon to fight the pandemic, including retired professionals and those who have just completed formal education. Additionally, at some sites, research personnel with relevant accreditation are assigned to patient care at COVID-19 clinics.
Running clinical trials at hospitals requires the oversight of a principal investigator; the involvement of collaborators, including research fellows; and the execution of trial activities by research staff. All might become unavailable due to duties associated with the pandemic, such as the need to remain at home for parental responsibilities or due to COVID-19 infection. Communication is pivotal. Principal investigators should ensure adequate trial oversight and communication with trial participants. The unavailability of the principal investigator should be reported to the coordinating centre and sponsor, and delegation or a designated replacement communicated to ethics committees and applicable local authorities.9,11,13,14
Reduced capacity at investigational sites will impact on availability to perform study visits (or phone calls) to assess and confirm eligibility, enter data in electronic case report forms (eCRFs), to report (serious) adverse events and to follow the protocol in general. All protocol deviations should be noted, with those that are pandemic related clearly identified. Most importantly, principal investigators must ensure that enrolled subjects fully comply with eligibility criteria and that all measures are taken to report adverse events in a timely fashion, given that these two are of paramount importance for patient safety. Coordinating centres may require an increased level of monitoring of eCRFs and a degree of flexibility in terms of timing for data cleaning.9,11,13,14
Impact on Coordinating Centres and Monitors
Large, multicentre collaborative trials require the participation of a coordinating centre, either a contract or an academic research organisation. Coordinating centres execute the study, or activities within, on behalf of the sponsor or manufacturer. A study team is composed of a project manager, clinical research associates and study monitors, data managers, biostatistician, quality assurance manager and safety reporting units, with or without a medical monitor. A pandemic prompts the need to work from home and cancel face-to-face visits. Where systems are upgraded to allow remote work and staff remain available for reception of materials, coordinating centres can continue to operate during a pandemic. Site initiation and monitoring visits are cancelled, postponed or performed remotely using web-based technology (although source data verification can be postponed). Remote monitoring is possible, but might not be feasible at all participating sites in a trial and increases the workload at the site. Moreover, technical requirements, confidentiality issues, updated consents and the increased burden to site personnel could make it impractical.13 In line with this, quality assurance measures, such as site audits, are postponed unless serious non-compliance is identified.
Impact on Trial Committees
The participation of several committees in clinical trials ensures proper scientific and operational oversight, data integrity and quality, as well as patient safety. Typically, the steering committee is composed of established investigators or key opinion leaders, and representatives from parties involved (e.g. coordinating centres, sponsor, grant givers). During the pandemic, office-based professionals work from home, and participation may be limited. Nevertheless, given the oversight duties of the steering committee and data and safety monitoring boards, the frequency of meetings might need to be increased to address immediate pandemic-related needs. At the beginning of the pandemic, cardiovascular clinicians saw a reduction in patient load, as the population was advised to stay at home. Unfortunately, this has resulted in late presentations of severe conditions (e.g. non-STEMI or decompensated heart failure). However, as hospital resources are depleted, not only in materials but also in personnel, cardiovascular clinicians are required to perform pandemic-related tasks and to self-isolate, potentially limiting their availability for participation in committees. The same applies to members of clinical event committees and data and safety monitoring boards. Potential exceptions are data managers and biostatisticians. In theory, this could reduce the availability of clinicians to participate in committee calls; however, in practice, this might not be the case. Committed investigators tend to stretch time when required, as shown by Chinese investigators who managed to report initial cohorts despite being at the centre of the pandemic.2,4 All meetings are planned as teleconferences.
Impact on Core Laboratories
Cardiovascular trials, particularly interventional trials, rely heavily on imaging. For the purpose of an unbiased and consistent analysis, central laboratories are utilised. Imaging modalities, such as echocardiography, ECG, cardiac MRI, angiography assessments, intracoronary imaging and cardiac CT, are frequently used. Thus, for a core laboratory to ensure timely delivery during a pandemic, conditions should allow analysts and supervisors to work remotely. Data should reach core laboratories electronically, with secure and certified data-transfer providers. Time windows for imaging follow-up might need to be adjusted and uploading activities may also be interrupted. Analysing cardiovascular images might not be as efficient at home when compared with a well-equipped work environment. However, remote access through a secure connection to software and datasets, as well as databases, will allow continuity of activities. Information and communication technology departments play a pivotal role in setting up and maintaining reliable infrastructure. A lack of remote access could force activities to stop during a pandemic.
Safety Oversight
Safety reporting should continue in line with national regulations and following standard procedures.9–11,13,14 Investigators should ensure timely capture of serious adverse events, a process that might involve extended use of telehealth. Moreover, serious adverse events should be identified, where possible, as pandemic or non-pandemic related. The inability to deliver investigational drugs could pose additional risks to participants and warrants an increased level of safety monitoring.9,10 Ongoing trials lacking data and safety monitoring boards might need to revisit that decision on a per-case basis. Data and safety monitoring boards may independently assess an ongoing trial that has been severely affected by the pandemic (e.g. incomplete data, incomplete follow-up) to help investigators and sponsors elucidate, without compromising the integrity of the trial, whether continuing the trial will yield interpretable data.12
Impact on Protocol Adherence and Trial Reporting
A pandemic has a significant impact on the ability to adhere to protocol requirements (e.g. missed follow-up visits or tests). Importantly, protocol deviations should be documented with an indication that they are pandemic-related following standard procedures.9,12–14 Data collection could be challenging, but should not stop. When reporting the results of a trial, cohorts might need to be divided as pre-pandemic, peri-pandemic and post-pandemic.12 Statistical analysis plans might need adaptions when considering the influence of the pandemic in the interpretability of results, especially when endpoints share characteristics with COVID-19-related events.9 Guidance on the interpretability of results when analysing data with missing values, unbalanced completeness or out-of-window assessments (e.g. echocardiograms, control angiograms, laboratory values) might also be required, depending on the duration of the pandemic. For multicentre trials, a per-site assessment might be required for outbreak areas versus non-outbreak areas. The interpretability of the overall evidence generated should be discussed with regulatory authorities.9,12–14 The use of vaccines, once available, might also require adequate documentation in study databases to avoid unbalanced usage.
Impact on Ethics Committees and Regulatory Agencies
Ethics committees (ECs; or institutional review boards [IRBs]) and regulatory agencies experience a significant increase in activity during a pandemic. ECs/IRBs face the burden of protocol amendments for ongoing trials, and prioritise activities related to the pandemic, including the review of COVID-19 trial submissions.9,12–14 Regulatory agencies play a critical role in protecting citizens from threats, including emerging infectious diseases, thus the importance of providing timely guidance, such as the regulatory documents that form the basis of this article.9–14 Based on accumulating experience, the advice of ECs/IRBs and regulatory agencies to sponsors and investigators could be critical to determine the continuation, modification or pause of trial activities. Such recommendations are complex, given the uncertainties related to the pandemic duration.
Conclusion
A pandemic has a significant impact on every component of cardiovascular clinical research. When facing a rapidly spreading disease with no effective treatment or vaccine, efforts should be focused on facilitating the day-to-day work of healthcare professionals with required personal protective equipment. Pandemic-related investigations should be prioritised. Nevertheless, sponsors and investigators should take all necessary actions to ensure patient (and employee) safety and to maintain trial integrity in ongoing, non-pandemic-related clinical trials, and capture pandemic-induced trial adjustments in focused amendments so that meaningful conclusions can be achieved when reporting results.