Acute heart failure (AHF) is a leading cause of hospitalisation in developed nations, responsible for 67,000 admissions a year in the UK alone, an incidence which is set to increase with an ageing population.1 The prognosis remains poor: in-hospital mortality ranges from 4–11 % and in those patients surviving to discharge 50 % will be re-admitted and one-third will die within 12 months.2–6 These poor outcomes indicate room for improvement in the standard of care. There may be several reasons why this is so and the failure to make an accurate and timely diagnosis may be an important contributor. There is no single diagnostic test, the symptoms and signs may be non-specific and may be further compounded by features of co-morbidity, meaning a high index of clinical suspicion is essential.7,8 These difficulties are set against a backdrop of concern amongst the profession about the declining status of the utility of bedside assessment in modern medicine.9,10 Failure to recognise suspected HF may delay major investigations, instigation of appropriate initial management and access to specialist input; all of which influence clinical outcome in the short and long term.1 Delayed diagnosis at presentation may result in patients being inappropriately transferred to non-specialist wards, resulting in longer stays, increased re-admission and poorer outcomes.11 Indeed, the wide variation in current management and service provision taken together with a lack of new treatment developments over the last 25 years has led to AHF being termed the ‘Cinderella’ of cardiology.11
It is of no surprise therefore that contemporary practice guidelines all highlight the importance of early recognition of HF in a patient presenting acutely to allow prompt access to those treatments and services with proven survival benefit.12–14 This short review focuses on the clinical signs and radiographic changes present in AHF and discusses their relative utility in the assessment and diagnosis of these patients alongside the most up to date evidence.
Defining ‘Acute Heart Hailure’ – A Spectrum of Clinical Syndromes
‘Acute heart failure’ has been criticised as an imprecise term that has consistently defied a consensus definition, with disparate interpretations across clinical medicine and the clinical trial environment.15 One recent definition is: “The rapid onset of a clinical syndrome where the heart is unable to pump adequate blood to provide for the needs of the body.”12 In UK clinical practice, the term is generally taken to describe the rapid onset of the symptoms and signs of HF occurring over hours to days; typically resulting in hospital admission. Conversely, AHF has also been used to in reference to any patient with HF requiring hospital admission. The latter term confusingly encompasses a different patient population by including those with a subacute or chronically progressive time course. Either may reflect a ‘decompensation’ of known HF, or a de novo presentation in a patient previously undiagnosed.
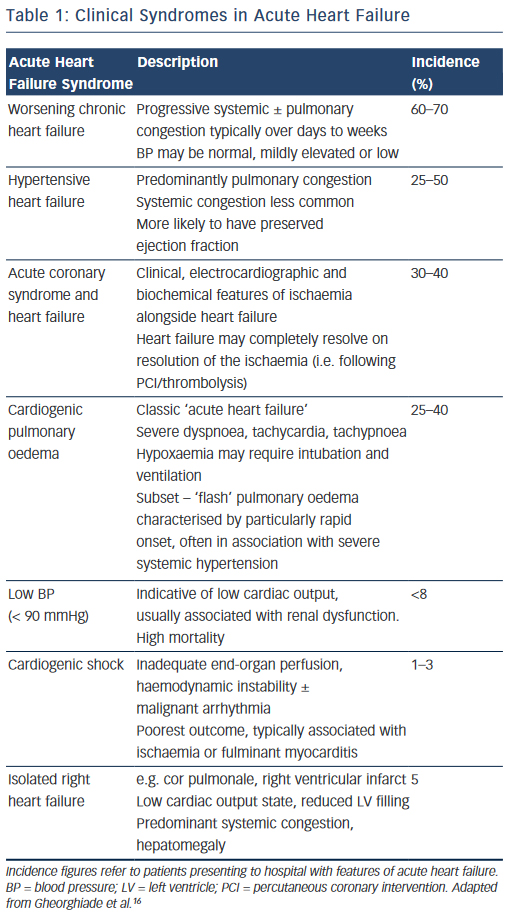
In reality, the clinical features of AHF result from a heterogeneous group of syndromes with significant overlap reflecting multiple underlying aetiologies, of which only around 50 % have a reduced left ventricular (LV) ejection fraction (EF) (see Table 1).16 The clinical severity may vary in accordance with the particular syndrome, (e.g. from predominant peripheral oedema to cardiogenic shock), or dependent on the individual patient; while certain presentations require specific treatments delivered in a time-critical manner (e.g. HF with an acute coronary syndrome). For optimal management, therefore, it is essential that the assessing clinician is able to elicit and interpret the clinical history and physical examination signs to highlight both the diagnosis, the likely underlying cause and potential precipitants.
Clinical Signs
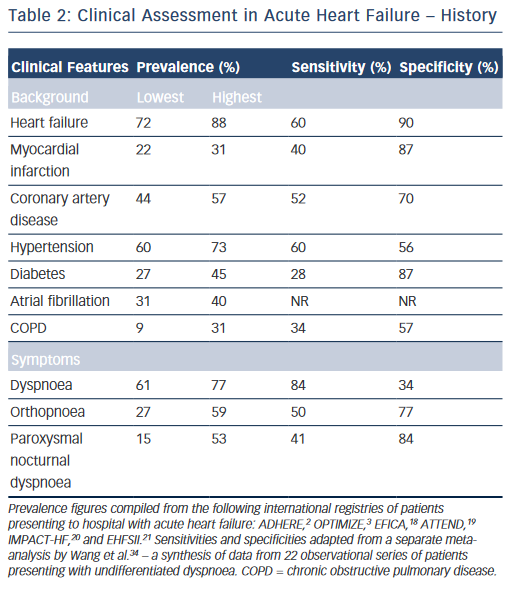
While the expected clinical features of a patient with AHF can be recounted by every competent medical student, their precise prevalence in clinical practice and relative discriminatory power in excluding other differential diagnoses have proven more difficult to quantitate formally. Clinical trials provide a wealth of high-volume information with accurate follow-up data, but limit their broader applicability by omitting a large proportion of ‘real world’ patients through strict inclusion criteria, typically excluding for example, the elderly and those with multiple co-morbidities.17 A number of large observational cohort studies have been undertaken over recent years in part to combat this shortcoming.2,3,18–21 The results are summarised in Table 2 and merit discussion below.
History
The background of patients presenting acutely with features of HF is largely consistent. They tend to be elderly, with a mean age of 60–65 in clinical trials, and 70–75 in observational series.2,3,18–23 Most registries identify a slight male preponderance (59–61%),18–21 although this is not always the case.2,3 In Western populations, most have a pre-existing history of HF (67–88 %).2,3,18,20,21 Other common co-morbidities include hypertension (60–73 %), diabetes mellitus (27–45 %), atrial fibrillation (31–40 %) and renal dysfunction (28–30 %).2,3,18–21 Chronic obstructive pulmonary disease (COPD), an important confounder in the differential diagnosis is also prevalent (9–31 %).2,3,18–21,24
Dyspnoea is the most common presenting symptom and is the most sensitive, although not entirely ubiquitous (prevalence 61–95 %) indicator of congestion.2,3,6,18–21 It should be categorised in accordance with an accepted scale (e.g. New York Heart Association functional classification).25 This documentation is important for ongoing management as the severity of dyspnoea predicts mortality and is stubbornly resistant to symptomatic control in a significant proportion of patients despite optimal therapy.26 While the prevalence of dyspnoea in other pathologies (e.g. COPD) means that it lacks sufficient discriminatory power in isolation, it should always act as a flag to the presence of AHF within the differential diagnosis, ranking high up the list in those patients fitting the above demographic and co-morbid profile. Orthopnoea (27–59 %) and paroxysmal nocturnal dyspnoea (15–53 %), the archetypal textbook symptoms of HF, typically reflect a more-severe presentation and are unsurprisingly less common but less ambiguous. However, they still lack the requisite specificity (77 and 84 %, respectively) for diagnosis when considered in isolation in patients presenting to the emergency department with undifferentiated dyspnoea (see Table 2).2,3,18–25
Other important but non-specific symptoms of HF include fatigue, wheeze, abdominal bloating, anorexia, confusion and weight change; although their precise prevalence in patients presenting acutely is less well characterised in the literature.13 A cough productive of pink frothy sputum is relatively uncommon.
The precipitating factor behind the presentation may also be gleaned from the history and should be actively sought. One or more precipitants were identified in 61 % of 48,612 patents with known HF presenting acutely in the US. These included respiratory infection (15 %), ischaemia/acute coronary syndrome (15 %), arrhythmia (14 %), uncontrolled hypertension (11 %), medication non-adherence (9 %), worsening renal dysfunction (7 %), diet non-adherence (5 %) and others. Nineteen per cent of patients had more than one precipitant to be addressed.27 In a smaller cohort (n=150) concentrating specifically on acute pulmonary oedema, the identified precipitants were similar including hypertension (58 %), ischaemia (38 %), infection (37 %), arrhythmia (28 %) and anaemia (21 %) among others.28
Physical Examination
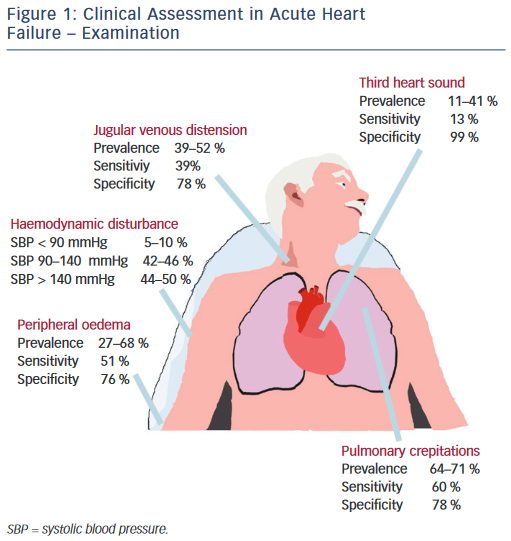
Physical examination centres on haemodynamic evaluation and assessing for the clinical signs of congestion (see Figure 1). Patients with AHF may have a high, low or normal blood pressure. Normotension or moderate hypertension is the most common finding in observational series (see Table 2).2,3,18–21 Uncontrolled severe hypertension, particularly in conjunction with predominant pulmonary congestion may indicate hypertensive HF, HF with preserved EF (HF-PEF), or ‘flash’ pulmonary oedema in extreme cases. Hypotension (5–10 %) is the least-common manifestation but marks an important adverse prognostic sign, typically reflecting compromised cardiac output that, dependent on the patient, should prompt the consideration of either more advanced therapies, such as vasoactive agents and mechanical support, or the need for palliation.2,3,18–21 The temperature of the extremities as assessed by palpation may also used as an indicator of cardiac output. Cool peripheries are present in up to 24 % of unselected patients.19 The Forrester classification system based upon whether patients have adequate circulation (warm or cold) alongside the presence or absence of congestion (wet or dry) is no longer included in current guidelines but provides a useful guide to applied haemodynamics.29,30
Assessment of the heart rate and rhythm will help to elicit important arrhythmias (e.g. atrial fibrillation), which may have triggered the presentation and will influence management. Other important ancillary signs, which indicate underlying structural heart disease, include cardiac murmurs. The most prevalent features of congestion are peripheral oedema (65–68 %) and bibasal crepitations or rales (64– 80 %), which in patients presenting to the emergency department with undifferentiated dyspnoea offer sensitivity and specificity of 51 and 76 % and 60 and 78 %, respectively.2,3,18–21
Measurement of the jugular venous pressure has been an integral part of bedside cardiology since its inception. In expert hands the value of jugular venous distension as an indicator of elevated right atrial pressure and its correlation with raised pulmonary capillary wedge pressure has been elegantly demonstrated in advanced chronic HF with excellent specificity.31,32 Yet even in this context the practical utility of this sign has not been universal, while in undifferentiated patients with acute dyspnoea sensitivity falls to as low as 39 %, perhaps reflecting in part a more general lack of confidence among the newer generation of clinicians in eliciting such signs.33–36 Similarly the third heart sound, a low pitched sound best heard over the left ventricular apex in conjunction with the rapid filing phase of ventricular diastole demonstrated excellent specificity and was an independent predictor of mortality in a large cohort of AHF patients in Japan.37 However, a lack of familiarity in the detection of this sign and poor inter-observer variability has been documented elsewhere, reflected in its low sensitivity (13–40 %) in patients with AHF presenting to the emergency department, even when augmented by acoustic cardiography (computerised measurement of the heart sounds with a synchronous electrocardiogram [ECG] trace using specialised electrodes placed at V3–V4).34,38–40
Special Considerations
Alongside an ageing population the demographics of HF patients are changing, e.g. changing cardiovascular risk profile (reduction in incidence of myocardial infarction) and developments in cardiac care (e.g. primary percutaneous coronary intervention). Some contemporary epidemiological series indicate the mean age at diagnosis may be as high as 80 years old.41 Patients in this category warrant special consideration and particular vigilance from the assessing clinician. Presentation may be subtle and more gradual, perhaps marked by atypical symptoms (e.g. anorexia and weight loss), with co-morbid conditions (e.g. musculoskeletal disease) acting as confounders to potentially decrease sensitivity.42 In contrast to their younger counterparts, elderly patients (>85 years) with AHF are more likely to be female and have a preserved EF.43 Atrial fibrillation, hypertension, anaemia, renal dysfunction and cerebrovascular disease are all more common – while diabetes and coronary artery disease are conversely less frequent – perhaps implying that patients with these conditions are more likely to present earlier.44 The expected typical examination findings are also less frequent compared with younger (<65 years of age) patients.45
The studies detailed above mostly refer to patients in Western Europe and the US. Outside of this demographic some main differences in the background and presentation of patients with AHF exist. In Eastern Europe, Asia, Africa and the Middle East, patients are much more likely to present acutely with de novo HF.46–50 Patients in Saudi Arabia are younger, with a higher incidence of diabetes and a greater preponderance for left ventricular systolic dysfunction.50 Patients in the Asian-Pacific are also younger, less co-morbid and tend to exhibit a more severe phenotype.49 Finally, across a range of sub-Saharan African countries the incidence of ischaemic heart disease is much lower than in western populations with hypertension the predominant HF aetiology.48
Chest Radiograph
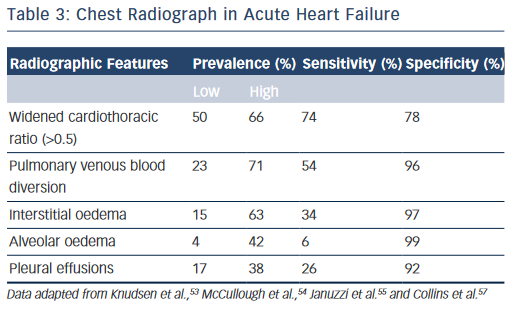
The chest radiograph is a low cost, easily obtainable investigation that should be routine in all acutely dyspnoeic patients with suspected HF; serving to exclude alternative pathologies (e.g. pneumonia, pneumothorax, lung cancer) and/or demonstrate features of HF.13,14 The classic radiographic features of HF have been elegantly described in conjunction with physiological haemodynamic data and are detailed in Table 3.51,52 Pulmonary venous blood diversion (PVBD), or cephalisation, results from redistribution of the pulmonary blood flow and typically occurs when pulmonary artery wedge pressure (PAWP) is greater than 10–15 mmHg. Interstitial oedema, characterised by peripheral septal (Kerley B) lines due to thickening of the interlobular septa, is thought to result in a PAWP greater than 20 mmHg, while alveolar oedema is present when PAWP exceeds 25 mmHg.52 However, this hierarchy of radiographic changes is not ubiquitous and may only be partially present, or indeed absent, even in cases of advanced HF.51
In the acute setting, when present in patients exhibiting the clinical features of HF outlined above, PVBD, interstitial oedema and alveolar oedema are all ‘high value’ signs; with specificities of up to 96 %, 97 % and 99 %, respectively (see Table 3).46,53–55 However, these signs are unsuitable as screening tools as sensitivity is poor for all three measures (see Table 3, respectively: PVDB 54 %, interstitial oedema 34 %, alveolar oedema 6 %) with a wide variability in prevalence.46,53–55 Increased cardiothoracic ratio was more common (prevalence 50–66 %) thus more sensitive but less specific (Table 3, 74 and 76 %, respectively).46,53–55 Nevertheless, the chest radiograph remains an imperfect tool for detecting cardiac enlargement, and a large observational series in the US demonstrated that up to 20 % of patients discharged with a diagnosis of AHF may have a completely ‘negative’ film with features of HF entirely absent.56,57 The above studies are all importantly limited by a lack of core laboratory analysis, and as such may reflect some diagnostic inaccuracy, although should certainly serve to caution physicians against excluding HF in symptomatic patients based on radiographic findings alone.
Integrated Assessment – How to Improve Time to Diagnosis
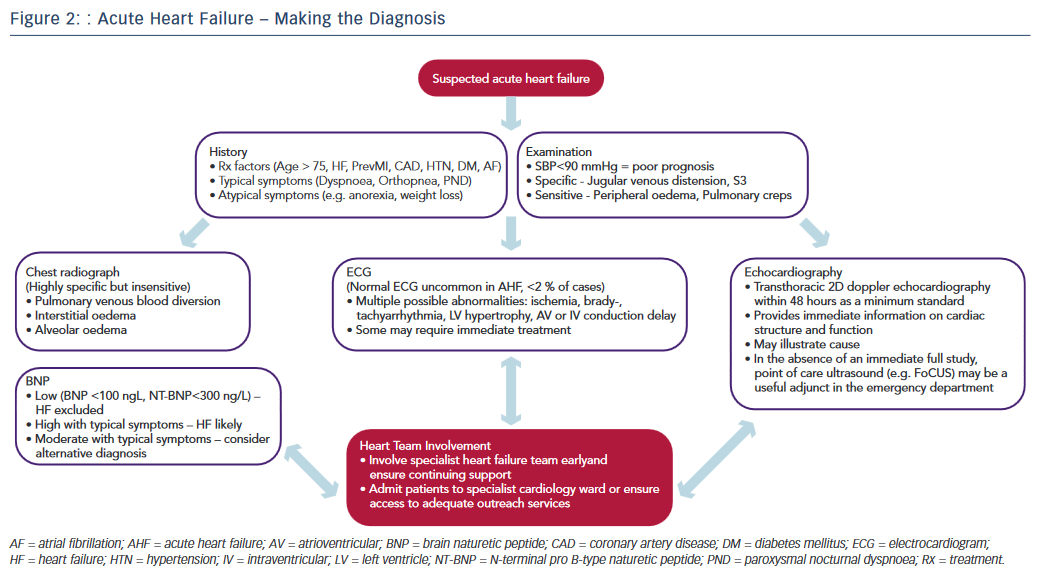
Recognising the diagnostic limitations of the clinical and simple radiographic parameters outlined above, contemporary practice guidelines emphasise the essential role of targeted investigations in identifying cardiac abnormalities to improve diagnostic accuracy.12–14 The pivotal role of the three most crucial investigations (ECG, echocardiography, biomarkers) is discussed below and illustrated in the diagnostic pathway of AHF (see Figure 2).
The ECG remains integral to the investigative armamentarium of the physician assessing a patient with suspected AHF, and may identify a host of abnormalities (e.g. brady- or tachyarrythmia, ischaemia, LV hypertrophy, atrioventricular or intraventricular conduction delay) to increase clinical suspicion and highlight precipitants that may require specific immediate management (reperfusion, pacing, rate control, anticoagulation, etc.) Indeed, an entirely normal ECG trace in AHF is highly unusual (<2 % of cases) yet not sufficient to independently rule out the diagnosis.58
Naturetic hormones are released by the heart in the presence of disease or in response to increased atrial or ventricular load. They lack the requisite specificity to diagnose HF in isolation, being secreted in other states such as arrhythmia, pulmonary embolism, renal failure, obesity and advancing age. However the sensitivity of the two most commonly used markers in clinical practice, brain naturetic peptide (BNP) and its precursor N-terminal pro B-type naturetic peptide (NT-proBNP), is such that a normal level (BNP <100 pg/L; NT-proBNP <300 pg/L) in the presence of suspected AHF effectively excludes the diagnosis.59 Moreover, a significant body of evidence highlights the magnitude of BNP/NT-proBNP elevation as an independent predictor of adverse outcome in AHF.60 Accordingly, obtaining BNP/NT-proBNP levels is now considered mandatory in suspected AHF.12 Furthermore, as familiarity with clinical use of biomarkers has increased, a pragmatism in their interpretation has continued to develop. For example, a patient with equivocal symptoms and signs and a modest elevation in BNP is liable to indicate HF; while severe symptoms combined with a mild elevation in BNP suggests an alternative diagnosis. Finally, recent work demonstrates that the use of multiple biomarkers in the elderly may augment diagnostic accuracy still further.61
Echocardiography provides immediate information on cardiac anatomy (chamber volumes and mass, valve structure) and function (ventricular wall motion, valvular function, pulmonary artery pressures) and plays an indispensable role in both the diagnosis of HF and guiding specialist management.13,14 The myriad potential echocardiographic abnormalities in AHF lie outside the scope of this article and have recently been discussed extensively elsewhere.62,64 Nevertheless it is important to emphasise that while the evidence base around the precise timing and modality of echocardiography in AHF requires further development, a transthoracic 2D doppler study within 48 hours is regarded as the minimum standard of care.12
Several pilot studies also highlight a potential emerging role for point of care ultrasonography in the emergency department as a supplementary tool to rapidly assess for filling status (e.g. inferior vena cava parameters) and features of congestion (e.g. sonographic B-lines) in the dyspnoeic patient.63,64 Moreover, once a diagnosis is reached further testing targeted at revealing the underlying aetiology (e.g. coronary angiography, myocardial perfusion scan, cardiac magnetic resonance) is usually required.
While none of the above tests demonstrate sufficient diagnostic accuracy to be a singular ‘gold standard’ in AHF, their prompt initiation alongside timely access to relevant specialist HF input are recognised as central priorities in improving the standard of AHF care.11 The most recent National Institute for Health and Care Excellence guidelines emphasise the importance of the provision of a specialist HF team (HF specialist nurses, consultant cardiologists specialising in HF, etc.) based on a cardiology ward with dedicated outreach services for all patients with suspected AHF, highlighting the consistent improved outcomes in national data sets from this approach.1,12 The key to improving the time to diagnosis and subsequent outcomes in AHF therefore lies in maintaining a high index of suspicion allied with an awareness of the limitations of clinical assessment and recognition of the requirement for major investigations combined with the appropriate organisation to deliver these and involvement of appropriate HF specialists in a practical and efficient manner (see Figure 2).
Limitations
The majority of the studies quoted above are based upon observational data and should be interpreted within the following important limitations. First, patient registries, while providing a wealth of high-volume data encompassing ‘real-world’ patients with few exclusion criteria, are often based on convenience, rather than consecutive samples and may employ heterogenous diagnostic criteria, particularly in HF where there is no recognised standard, which in both cases can introduce selection bias. Second, utilising the primary discharge diagnosis in order to identify cases may introduce a proportion of patients presenting emergently with another condition who subsequently went on to develop AHF during their hospital stay or conversely exclude patients for which HF was only a co-morbid diagnosis (e.g. myocardial infarction complicated by HF). Finally, the retrospective review of medical records, even when conducted alongside standardised methods is dependent upon the quality and accuracy of the original data entered.
Conclusions
This review has served to highlight the key presenting characteristics of patients with AHF based upon the most recent observational data. Much information can be gleaned from the background history with a significant cardiac history and common risk factors (e.g. hypertension) frequently present. Of the presenting symptoms, dyspnoea is sensitive but not specific, while the more classic symptoms (orthopnoea, paroxysmal nocturnal dyspnea) are higher value but frequently absent. Typical examination findings (jugular venous distension, third heart sound) and radiographic features (PVBD, interstitial oedema, alveolar oedema) are also highly specific but insensitive.
More crucially, the above data illustrate that patients with AHF are a heterogenous group, compounded by imprecise diagnostic criteria and co-morbidity (COPD, musculoskeletal disease, cognitive impairment), which may mask important clinical features. The key to improving the time to diagnosis in AHF rests upon a high index of clinical suspicion alongside a readiness to initiate relevant additional tests (biomarkers, echocardiography) and facilitate the early involvement of specialist care.