Heart failure with reduced ejection fraction (HFrEF) and AF share several predisposing risk factors and often coexist in the same population.1 Although AF can be a marker of worsening heart failure (HF), it can also be a main driver of disease progression. The presence of AF in patients with HFrEF is associated with an increased risk of stroke, re-hospitalisations and all-cause death.2 Therefore, restoration and maintenance of sinus rhythm was initially thought to be preferable in HFrEF patients, in whom atrial systole may play a critical role in left ventricular filling and overall haemodynamics.3–5
Surprisingly, large studies have failed to prove a significant difference in cardiovascular outcomes between rate and rhythm control-based strategies in the HFrEF population.6 This may be due, in part, to the limited choice of antiarrhythmic drugs for pharmacological cardioversion that can be used in HFrEF.5 As a result, current clinical guidelines favour a rate control strategy for patients with AF and HFrEF over rhythm control.7 However, in the past few years the use of catheter ablation for the definitive treatment of AF has been investigated in comparison to medical treatment of AF. An overall benefit with AF ablation seems to be present compared with both pharmacological rhythm and rate control.8 Here, we review the most recent results in the literature comparing catheter ablation with pulmonary vein isolation (PVI) and pharmacological treatment of AF with amiodarone.
Use of Amiodarone in Patients with AF and Heart Failure with Reduced Ejection Fraction
Although guidelines still favour a pharmacological rate control approach as first-line therapy in patients with AF, there is a Class IIa recommendation for AV node ablation or rhythm control in patients with chronic HF who remain symptomatic from AF despite a pharmacological rate control strategy.7 Based on the 2014 American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Rhythm Society (HRS) guidelines, only two antiarrhythmic medications are recommended for the treatment of AF in HFrEF: dofetilide and amiodarone.7 Other antiarrhythmic drugs, such as Class Ic agents, should be avoided because of their possible proarrhythmic and negative inotropic effects.
Although dofetilide seems effective in restoring sinus rhythm and reducing rehospitalisation, it failed to show a mortality benefit, possibly because of proarrhythmic effects in patients with QTc prolongation.5,9 Amiodarone represents the most effective medication for rhythm control and it is by far the most used in HFrEF patients.10 Despite its efficacy, amiodarone is associated with organ toxicity, such as liver failure, thyroid dysfunction and pulmonary fibrosis, which has limited its use, especially as a maintenance therapy.11 In addition, a subanalysis of the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) showed that, compared with placebo, amiodarone did not provide a mortality benefit in AF patients with a left ventricular ejection fraction (LVEF) <35% and New York Heart Association (NYHA) Class II/III.12 The trial further suggested an increase in non-cardiac mortality with amiodarone in patients with NYHA Class III HF. These results should be interpreted with caution because they represent a study subanalysis in a selected patient population with HFrEF and NYHA Class III.12 Nevertheless, they raise concerns on the risk–benefit balance of amiodarone use in patients with advanced HF.
Furthermore, amiodarone failed to show a significant benefit compared with rate control in HFrEF.6 The Atrial Fibrillation and Congestive Heart Failure trial (AF-CHF) was a large multicentre study that randomised 1,376 patients to either rate control or rhythm control, of whom 80% were treated with amiodarone. The main finding of the study was that rhythm control was associated with an increased rate of hospitalisation and had no mortality benefit.6
Clinical Benefits of Catheter Ablation Over Rate Control Strategies
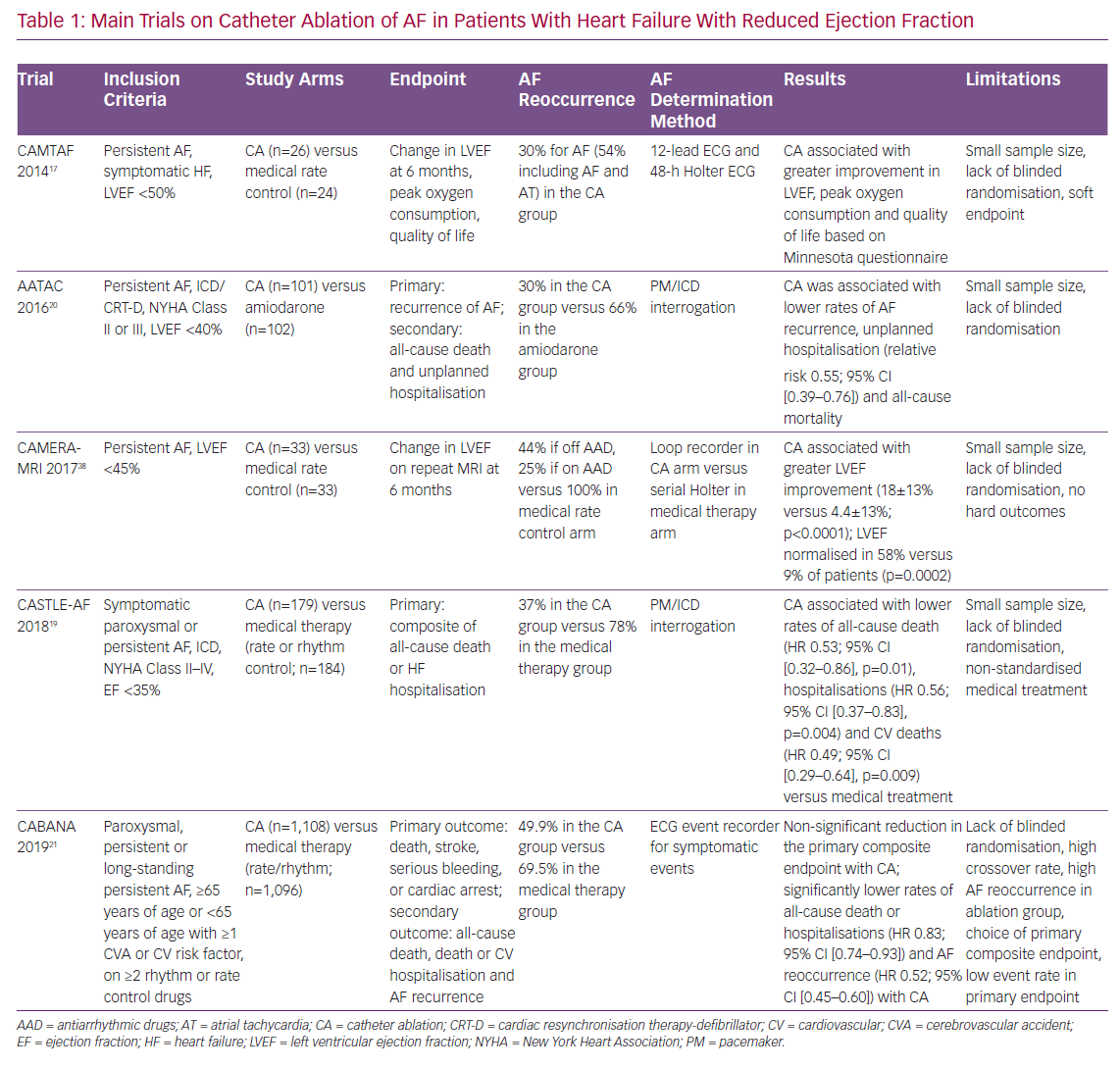
Given these limitations of pharmacological rhythm control strategies, attention has been shifted towards catheter ablation for the definitive treatment of AF (Table 1). In 2014, catheter ablation received a Class IIb recommendation from the AHA/ACC/HRS for selected patients requiring rhythm control who were not suitable for or refractory to pharmacological therapy.7 Similarly, the recently updated 2019 guidelines provide a Class IIb recommendation for catheter ablation in patients with symptomatic AF and HFrEF because of its potential benefit in both mortality rate and rehospitalisation for HF.13
These guidelines are based on a handful of trials overall showing improved LVEF and long-term clinical outcomes with catheter ablation of AF in HFrEF patients compared with sole rate or rhythm control.8 These results are somewhat unsurprising, given that AF is the most common cause of tachyarrhythmia-induced cardiomyopathy, and that even short runs of tachycardic AF can trigger an acute decompensation in patients with an already compromised systolic function.14 In the long run, persistent AF can chronically worsen LVEF, a phenomenon that seems to occur even with normal heart rates, likely due to asynchrony and irregular heart rhythm.15
The Comparison of Pulmonary Vein Isolation Versus AV Nodal Ablation With Biventricular Pacing for Patients With Atrial Fibrillation With Congestive Heart Failure (PABA CHF) trial showed that PVI was superior to atrioventricular node ablation with biventricular pacing in patients with HFrEF and uncontrolled AF with regard to improved cardiac function, exercise capacity and quality of life.16 Interestingly, the PABA CHF trial showed that heart rate control with atrioventricular node ablation and pacemaker (PM) implantation is not as effective as AF catheter ablation with restoration of sinus rhythm in improving LVEF, stressing the importance of long-term effective rhythm control over rate control.
The results of the PABA CHF trial were confirmed by the Catheter Ablation Versus Medical Treatment of AF in Heart Failure (CAMTAF) trial, a small study on 50 patients with HFrEF randomised to either catheter ablation or pharmacological rate control.17 In that study, a significant improvement in ejection fraction was found at 6 months in the catheter ablation group with an 80% arrhythmia-free survival rate. Quality of life, assessed by means of the Minnesota Living With HF Questionnaire, was also improved in the catheter ablation arm.17 Contrary to prior studies on pharmacological rhythm control versus rate control, these trials on catheter ablation did show a significant benefit in maintaining sinus rhythm in HFrEF patients over simple rate control of AF. In addition to advocating for the safety and efficacy of catheter ablation, these results highlight possible limitations of pharmacological rhythm control.
Direct Comparison of Catheter Ablation of AF and Pharmacological Rhythm Control
Evidence on the efficacy of catheter ablation of AF in HFrEF patients compared with pharmacological rhythm control has been increasing in the past few years. Interpretation of such evidence has been difficult because of the heterogeneity of the study populations, the pharmacological treatment strategies used, the methods for the determination of AF reoccurrence, etc. Even the degree of expertise of the centres performing the ablation represents a significant source of variability among trials. Nevertheless, the use of catheter ablation of AF in HFrEF patients seems to be more effective than pharmacological rhythm or rate control with respect to both soft endpoints, such as improved ejection fraction and hard endpoints, such as rehospitalisations and mortality rates.8,18
One of the main trials to affect the most recent guidelines on the management of AF is the Catheter Ablation versus Standard Conventional Therapy in Patients with Left Ventricular Dysfunction and Atrial Fibrillation (CASTLE-AF) trial.19 CASTLE-AF tested the use of catheter ablation of AF in patients with HFrEF and symptomatic paroxysmal or persistent AF who were not responding to antiarrhythmic drugs or had significant side-effects from the medications. Compared with medical treatment, catheter ablation was associated with a significant reduction in the risk of the composite endpoint comprising all-cause death or hospitalisation for worsening HF. Although the primary composite endpoint was mostly driven by reduction in rehospitalisation, a significant improvement in all-cause mortality and, in particular, cardiovascular mortality became evident after 3 years of follow-up.
Unfortunately, the control arm of CASTLE-AF included a heterogeneous group of patients treated with either rhythm or rate control pharmacological strategies, which complicates the interpretation of the results. The medical treatment was at the discretion of the clinician and, therefore, not standardised. Nevertheless, approximately 55% of the trial population had received amiodarone: in 45–47% of these patients, amiodarone failed to adequately control AF, whereas in 12–14% unacceptable side-effects from the medication were reported. Interestingly the benefits of catheter ablation were observed regardless of the use of amiodarone.19
Probably the main limitation of the CASTLE-AF trial is the study population itself. By enrolling patients who could not tolerate or failed medical treatment, patients were selected who were likely to benefit from any additional intervention to control AF. Nevertheless, CASTLE-AF represents a critical trial to prove the efficacy of catheter ablation in HFrEF. Furthermore, the findings of CASTLE-AF are not an isolated occurrence.
Similar results were obtained in the Ablation vs Amiodarone for Treatment of Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted ICD/CRTD (AATAC) trial in a broader population of HFrEF patients who did not previously fail medical treatment.20 The AATAC trial is also one of the few randomised studies to compare catheter ablation with a pharmacological arm comprising 100% amiodarone-treated patients.20 Inclusion criteria for AATAC included HF with an ejection fraction <40% and the presence of dual-chamber ICD or CRT device. Similar to CASTLE-AF, the mandatory presence of an ICD or PM in patients in the AATAC trial allowed for very accurate monitoring of AF reoccurrence during follow-up. The main finding of the AATAC trial was that catheter ablation of AF was superior to amiodarone in achieving freedom from AF at the 2-year follow-up, as determined by PM interrogation. Importantly, catheter ablation of AF was associated with a significant reduction in unplanned hospitalisation for HF and overall mortality compared with amiodarone treatment.20
Most recently the Catheter Ablation vs Anti-arrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial produced similar results on a large population of 2,204 patients with paroxysmal, persistent or long-standing persistent AF.21 Although the rates of the composite primary endpoint of death, stroke, serious bleeding or cardiac arrest did not differ significantly between the catheter ablation and medical treatment (rate or rhythm) arms, likely because of the heterogeneity of the chosen endpoint components and a paucity of events, the rates of the secondary endpoint of all-cause death or rehospitalisation was again lower in the catheter ablation arm. This result from an intention-to-treat analysis is even more impressive because it occurred despite a 27% crossover rate from the medical treatment to the catheter ablation arm and a much higher AF reoccurrence rate in the catheter ablation arm (49%) compared with previous trials. Interestingly, CABANA also recorded data on symptomatic improvement and quality of life at 12 months. Using both the Atrial Fibrillation Effect on Quality of Life summary score and the Mayo AF-Specific Symptom Inventory frequency score, catheter ablation was found to be associated with significant improvements in symptoms and quality of life compared with the pharmacological arm of the study.22
Similarly, the Catheter Ablation Compared With Pharmacological Therapy for Atrial Fibrillation (CAPTAF) trial showed greater improvement in quality of life at 1 year with catheter ablation as measured with the General Health subscale score (Medical Outcomes Study 36-Item Short-Form Health Survey).23 It should be noted that patients’ self-assessment may be biased based on the treatment received. As in all trials on catheter ablations, sham procedures were not performed. Nevertheless, these results further support the safety of catheter ablation and its long-lasting clinical benefit over pharmacological approaches. Interestingly, although most clinical trials used radiofrequency ablation, cryoablation has also been considered. Few reports are available.
A recent study on 89 patients, 30 with HFrEF, noted lower success rates for PVI in patients with HF due to difficult cannulation of the right inferior pulmonary vein (PV), possibly because of the enlarged atrium.24 The recurrence of AF at 1 year was approximately 67% in patients with HFrEF after cryoablation.24 Data from randomised studies are not yet available; however, Ablation of Atrial Fibrillation in Heart Failure Patients (CONTRA-HF; NCT03062241), an on-going randomised multicentre trial, is testing the safety and efficacy of cryoablation versus guideline-recommended medical management in patients with HF. Although CONTRA-HF will not directly compare radiofrequency versus cryoablation in the HFrEF population, it will provide important information on the feasibility of this technique and the arrhythmia-free survival rate compared with optimal pharmacological treatment.
Limitations of Amiodarone for Rhythm Control
Although rhythm control with pharmacotherapy does not seem to improve outcomes compared with rate control in patients with concomitant HFrEF and AF, the use of a catheter ablation strategy seems to improve left ventricular haemodynamics and overall outcomes. A possible explanation would be an incomplete efficacy of pharmacological strategies in permanently maintaining sinus rhythm.
In the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study, a variety of medications could be used for rhythm control, but amiodarone was used in 62% of patients at some point during the study.25 At 5 years, only 63% of patients in the rhythm control arm were actually in sinus rhythm; this does not account for subclinical episodes of AF that may have occurred between follow-up visits. In addition, approximately 34% of the rate control population actually achieved and seemed to maintain sinus rhythm at 5 years.25
Similarly, in the AF-CHF trial, one of the largest randomised trials to show no benefit of pharmacological rhythm control over rate control in HFrEF patients, approximately 21% of patients in the rhythm control arm crossed over to rate control because of an inability to maintain sinus rhythm. Furthermore, it was estimated that approximately 56% of patients in the rhythm control arm had at least one episode of AF during follow-up.6 This percentage is likely to represent an underestimation, given that reoccurrence of AF was determined only with 12-lead ECGs during the scheduled follow-up appointments or by chart review. A much-needed subanalysis of the AFFIRM trial by the presence or absence of sinus rhythm showed a significant mortality benefit with maintenance of sinus rhythm.10 Most strikingly, antiarrhythmic drugs not only failed to improve survival, but were also associated with increased mortality after adjustment for the presence of sinus rhythm. The authors concluded that any beneficial antiarrhythmic effect of pharmacotherapy is offset by its adverse effects.10
Drug interaction in the setting of polypharmacy and medication non-compliance represent other possible contributors to pharmacological failure in this population.
Is Pulmonary Vein Isolation the Answer?
Although studies in favour of catheter ablation for the treatment of AF in HFrEF are slowly piling up, this approach is not free of limitations. As mentioned previously, what makes catheter ablation more effective than pharmacological rhythm control is essentially twofold: higher efficacy in restoring or maintaining sinus rhythm and avoidance of medication-induced side-effects. However, in the CASTLE-AF trial only 63% of patients in the ablation group remained in sinus rhythm during follow-up.19 Similarly, Jones et al. reported a single-procedure success of 68% at 1-year follow-up; when all patients undergoing a second procedure were included, the 1-year success rate increased to 88%.26
It is well-known that the recurrence of AF after catheter ablation is highly variable depending on the expertise and volume of procedures at the performing centres. In contrast with other cardiovascular procedures, AF ablation has not yet been standardised: some operators perform only PVI, whereas others pursue more aggressive ablation strategies, involving non-PV triggers. Non-PV triggers can potentially be identified in the left atrial posterior wall, the interatrial septum, mitral and tricuspid periannular regions, the crista terminalis and Eustachian ridge, the left atrial appendage, the coronary sinus or even the inferior vena cava and additional complex fractionated atrial electrograms.27–29
Although we may be unable to reach a one-size-fits-all approach for AF ablation, it is becoming evident that PVI alone is not sufficient to obtain long-term arrhythmia-free survival in almost 30–40% of patients.30 This is especially critical in the HFrEF population and in those with long-standing AF in whom AF ablation with PVI has a lower success rate than in those without HF or with only paroxysmal AF. HFrEF patients usually exhibit a severely diseased atrial substrate that can harbour a greater number of non-PV foci that ultimately account for the increased rates of AF and atrial tachycardia recurrence after sole PVI. Both reduced ejection fraction and the presence of non-PV triggers identified during the procedure are independent predictors of AF recurrence.31
Not surprisingly, several studies have shown that ablation of non-PV triggers significantly increases arrhythmia-free survival. For example, a subanalysis of the AATAC trial showed that when stratified by procedure type (PVI versus PVI plus non-PV triggers), success rates were significantly higher in patients treated with both PVI and non-PV triggers than those treated with PVI alone or amiodarone (p<0.001).32 Interestingly, when looking at outcomes, no benefit was found with PVI only versus amiodarone, suggesting that the encouraging results of the AATAC trial, in terms of both arrhythmia-free survival and hard outcomes, are driven by definitive restoration of sinus rhythm with aggressive ablation procedures including both PVI and non-PV trigger ablations. In patients with HFrEF, in whom strict sinus maintenance can, indeed, improve outcomes compared with incomplete rhythm control with pharmacotherapy, studies aiming to establish more comprehensive ablation strategies may result in further improvement in clinical outcomes.
To this end, stimulation protocols with adenosine and/or isoproterenol after PVI can successfully unmask non-PV triggers and guide further ablations, thus improving procedural outcomes.33 However, standardised stimulation protocols for residual triggers after PVI are also lacking.
Studies have shown that low doses and/or an incremental infusion of isoproterenol are not very effective in unmasking non-PV triggers, especially when AF ablation is performed under deep sedation or general anaesthesia. Conversely, the use of high doses of isoproterenol while the patient is in sinus rhythm seems to be associated with better yield of non-PV triggers and overall lower likelihood of arrhythmia relapse.34 In some cases, even in the absence of identifiable non-PV foci, patients with HFrEF could benefit from the empirical ablation of areas, such as the vena cava and the left atrial appendage.35–37
Conclusion
Catheter ablation of AF is more effective in restoring and maintaining sinus rhythm than pharmacological rhythm control with amiodarone. Most importantly, growing evidence suggests that catheter ablation is associated with a significant reduction in HF rehospitalisation and mortality likely because of a more stable, long-term maintenance of sinus rhythm and avoidance of side-effects of antiarrhythmic medications. Further large randomised clinical trials are warranted to confirm these results and to establish appropriate ablation strategies to maximise arrhythmia-free survival in HFrEF patients.