The most common cause of heart failure with reduced ejection fraction (HFrEF) in the industrialised world is coronary heart disease.1 Patients with an ischaemic aetiology of left ventricular systolic dysfunction have significantly higher mortality rates than those with non-ischaemic aetiologies.2 This more aggressive course represents the convergence of ischaemic myocardial fibrosis and endothelial dysfunction, which are superimposed on the progressive nature of the left ventricular dysfunction often with comorbidities such as diabetes and hypertension. The foundation of treatment for HFrEF is guideline- directed medical treatment.3 This is associated with a significant improvement in survival and quality of life, but not a return to normal activities. The most commonly considered surgical interventions for patients with HFrEF are coronary artery bypass surgery, sometimes combined with surgical ventricular reconstruction (SVR) and surgery for mitral regurgitation.
Percutaneous coronary intervention (PCI) has been less well studied in this setting. In a recent retrospective study from Alberta, Canada, Nagendran and colleagues identified 2,925 patients with coronary artery disease and left ventricular dysfunction (ejection fraction <35 %) of whom 1,326 underwent surgery and 1,599 received PCI.4 In a Cox proportional hazard analysis of the propensity-matched subgroups, surgery resulted in significantly lower rates of repeat revascularisation and better survival rates compared with PCI at 1, 5, 10 and 15 years. Following the SYNTAX study, the recommendation for patients with multivessel disease is invariably surgery and not PCI especially if the SYNTAX score is >30.5 Transplantation and left ventricular assist devices are indicated in highly selected patients with advanced disease.6 In patients with HFrEF who have coronary artery disease the essential question is whether flow-limiting obstructions should be treated with coronary artery bypass surgery.
About 30 years ago two celebrated randomised trials of coronary artery surgery were carried out. By modern standards of statistical evaluation the results should probably be considered neutral. Each trial managed to find a subgroup, usually not pre-specified, with a positive result. The Coronary Artery Surgery Study (CASS) noted that a subgroup of 78 patients with three-vessel disease and a left ventricular ejection fraction (LVEF) of 35–50 % had a 5-year mortality rate of 10 % where assigned to surgery and 19 % where assigned to medical treatment, which rose to 12 % and 35 %, respectively, at 7 years (p=0.009).7 Most of these patients had angina, few had heart failure and patients with an LVEF <35 % were excluded. The Veterans Administration Coronary Artery Bypass Study included 325 patients with impaired left ventricular function but excluded patients with ‘serious heart disease’.8 The 5-year mortality rates were 20 % with surgery and 27 % with medical treatment (non-significant difference). The 7-year mortality rates were 26 % with surgery and 37 % with medical treatment (p=0.049) and 47 % and 51 %, respectively, at 11 years (non-significant). A meta-analysis including 549 patients suggested an approximate 40 % reduction in mortality rate (p=0.02) in patients with left ventricular dysfunction compared with those with preserved ventricular function.9 But again, most of these patients had angina and few had developed heart failure.
In the absence of appropriate randomised controlled trials for revascularisation of patients with HFrEF, outcomes from observational studies have been used to make decisions in clinical practice. Such studies influence the outcome with treatment, which depends on a complex mixture of the effects of the intervention, the skills of the surgeon and the process by which patients are selected for treatment. This last factor is probably the most important. Results from an observational study in 2002 indicated that revascularisation might reduce mortality rates by 80% in patients with extensive viable myocardium but with no effect in those without extensive viability.10 Kunadian and co-workers conducted a meta-analysis of observational studies including 4,119 patients with a mean age of 64 years and an LVEF of approximately 25 % in those receiving surgical revascularisation.11 The perioperative mortality rate was around 5 %, which is similar to the 4 % noted in the Surgical Treatment for Ischaemic Heart Failure (STICH) trial.12 The observed 5-year survival rate was 73 % which is similar to that of modern medical treatment in a similar age group and much better than that seen in patients who received coronary intervention in either of the earlier trials of revascularisation.7,8 This shows that surgeons can select patients who can be operated on with reasonable results but does not show whether the outcome is better or worse than with medical treatment alone.
Unfortunately, recent randomised trials that have examined treatment for coronary artery disease that included an intensive medical regimen, such as the Medicine, Angioplasty or Surgery Study (MASS) ll and the Clinical Outcome Utilising Revascularisation and Aggressive Drug Evaluation (COURAGE) trial, excluded patients with severe left ventricular dysfunction.13,14 The Bypass Angioplasty Revascularisation Investigation in Type 2 Diabetes (BARI 2D) trial included patients with left ventricular dysfunction but only enrolled 17.5 % with an LVEF <50 %.15 The International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) is still in progress but excludes patients whose LVEF is <35 %.16
The STICH Trial
The STICH trial enrolled 1,212 patients with suitable coronary anatomy and an LVEF <35 % regardless of myocardial viability.12 Unfortunately, there was no genuine clinical equipoise between surgery and medical treatment. In those enthusiastic units that strongly advocated coronary artery surgery for heart failure, patients who were ‘good’ candidates for surgery including SVR received an operation and were not recruited into the trial. Moreover, as the trial progressed and recruitment became ever more difficult the entry criteria were subtly changed. This resulted in a trial not so much for heart failure as for ischaemia.
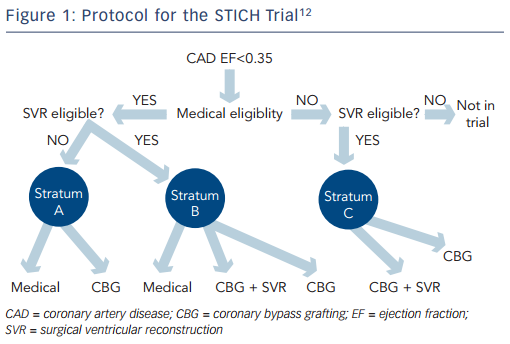
Nevertheless, the STICH trial is the only prospective, randomised controlled trial to specifically investigate the role of coronary artery surgery in patients with an LVEF <35 % who were also receiving guideline-directed medical treatment (for the trial protocol see Figure 1). The trial tested two hypotheses among patients with an LVEF <35 % who were suitable for coronary artery bypass surgery. The surgical revascularisation hypothesis evaluated surgery compared with medical treatment alone (n=1,212) and the surgical ventricular reconstruction hypothesis compared surgery with and without SVR (n=1,000). Patients with Canadian Heart Association Functional Class lll or lV angina and those with left main stem stenosis >50 % were excluded from the surgical arm, but were eligible for the SVR arm. Medical treatment included renin–angiotensin–aldosterone system inhibitors, beta-blockers, statins and antiplatelet agents titrated to optimal doses; diuretic drugs and digoxin were also used. Where possible, surgical treatment included at least one pedicled internal mammary conduit; this was accomplished in 91 % of patients.12
The Revascularisation Hypothesis
The 1,212 patients in the revascularisation hypothesis arm of the trial were enrolled in 99 sites in 22 countries.12 Using an intention-to- treat analysis, no significant difference was observed for the primary outcome of all-cause mortality between patients randomised to surgery versus medical treatment over a median follow-up period of 56 months. Of note, the surgery group had improved rates of death from cardiovascular causes and improved rates of a combined end- point of death from any cause plus admission to hospital for heart failure, which were pre-specified secondary outcomes.
Furthermore, Mark and colleagues showed that coronary artery surgery resulted in greater improvement in symptoms and quality of life than optimal medical treatment alone.17 Blinded end-point adjudication and mode of death analyses in the STICH trial revealed that surgery exerted a benefit across all common causes of death and death from myocardial infarction.18 These findings reveal an overall benefit of surgery against the background of modern medical treatment in patients with severe ischaemic left ventricular dysfunction.
STICH Subset Analysis
The initial target of this analysis was myocardial viability assessment, which was prospectively programmed into the design of the STICH trial.12 Multiple observation studies and meta-analyses had suggested that viability testing may be a powerful tool that will not only predict improvement in left ventricular function after coronary artery bypass surgery, but will identify patients with coronary artery disease and HFrEF with the greatest survival benefit from surgery compared with guideline-directed medical treatment.10,19 These studies were limited by their retrospective design, heterogenous methodology to define viability, lack of adjustment for key baseline variables (e.g. age and comorbidity) and the potential for selection of patients for surgery to have been influenced by the results of viability testing. Above all, these studies were performed before the modern era of medical treatment with few patients receiving beta-blockers.
In the original trial design, viability testing with single-photon emission computed tomography was required for entry to the study. But due to low recruitment, the protocol was revised to make single-photon emission computed tomography or viability testing with low-dose dobutamine echocardiography optional but strongly encouraged.20 Thus only 601 of the 1,212 patients enrolled in the revascularisation hypothesis arm underwent assessment of myocardial viability. The viability analysis did not identify patients who would preferentially benefit from surgery. Unsurprisingly, over a median 5.1-year follow-up, patients with viable tissue had a lower mortality of 37 % versus 51 % in patients without myocardial viability. However, after adjustment for other prognostic variables, myocardial viability was not associated with improved survival suggesting that patient comorbidities and the severity of left ventricular remodelling are more important determinants of survival. Furthermore, there was no significant interaction with respect to mortality between viability status and assignment to surgery or medical treatment (p=0.53).
The lack of a significant interaction between viability and survival with surgical versus medical management of patients with severe ischaemic left ventricular dysfunction is reflected in the 2013 American College of Cardiology/American Heart Association Guidelines for the Management of Heart Failure.6 These guidelines indicate that in the absence of angina, coronary bypass surgery may be considered for improving survival in patients with ischaemic heart disease who have severe left ventricular dysfunction (LVEF <35 %) whether or not viable myocardium is present (class llb, level of evidence B). Although the primary viability assessment in the STICH trial was on the basis of a pre-determined dichotomous separation of patients with viable versus non-viable myocardium, a separate analysis in which the viability data were analysed as continuous variables also failed to show an association between myocardial viability and improved survival with surgery.21
Further analysis of imaging data in the STICH trial was performed to assess inducible myocardial ischaemia in 399 of 1,212 patients in whom stress imaging was performed using single-photon emission computed tomography or dobutamine echocardiography. This analysis failed to show enhanced survival with surgery in any subgroups.22 This was unexpected and ran counter to the prevailing wisdom from observational studies and previous trials in patients with normal left ventricular systolic function or less severe left ventricular dysfunction. Similarly, circulating levels of brain natriuretic peptide and soluble tissue necrosis factor alpha receptor-1 were strongly related to survival in both the surgical and medical cohorts, but did not identify those with a survival advantage with surgery.23
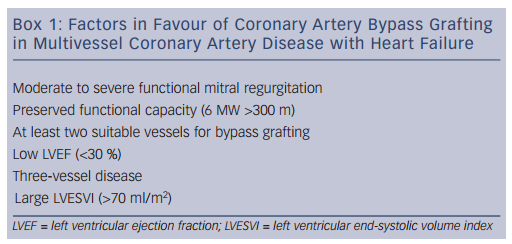
These data suggest that the observed survival benefits of coronary artery bypass surgery in patients with severe left ventricular dysfunction are driven primarily by factors other than biomarkers or objective markers of myocardial viability and ischaemia. Factors associated with higher survival rates with surgery include functional status as assessed by a 6-minute walk and the interaction of the angiographic severity of coronary artery disease, severity of left ventricular systolic dysfunction and severity of adverse left ventricular remodelling as assessed by end-systolic volume index. Patients with preserved effort tolerance but with multivessel disease, lower ejection fraction and higher left ventricular end-systolic volume index were most likely to benefit from surgery with respect to long-term survival (Box 1).
Ventricular Reconstruction Hypothesis
In patients with HFrEF there are often changes in left ventricular structure and function, which include remodelling of the left ventricle from its normal elliptical shape to a more spherical shape, resulting in a less efficient ventricle and a worse prognosis. SVR has been shown in observational studies to reverse-remodel the left ventricle and restore some of its original functional capacity.24,25 The procedure involves removing akinetic or dyskinetic segments of the anterior wall and reshaping the left ventricle to restore its original elliptical form. These techniques were developed from operations for left ventricle aneurysms by Vincent Dor26 and others.
It was uncertain from these observational studies whether SVR combined with coronary artery bypass surgery would result in improved outcomes for patients with ischaemic cardiomyopathy compared with coronary bypass surgery alone, especially when combined with medical treatment. This led to the design of the SVR arm of the STICH trial, the only randomised trial to question the role of SVR in patients with HFrEF. Patients were eligible if they had significant coronary stenosis amenable to surgical revascularisation, severe systolic dysfunction with an LVEF <35 % and dominant left ventricular anterior akinesia or dyskinesia amenable to SVR. A total of 1,000 patients were randomised to the arm comparing isolated coronary bypass surgery versus coronary bypass and SVR against the background of medical treatment. Primary outcome was a composite of all-cause mortality and hospital re-admission.
Patients who underwent SVR had significantly lower LV volumes on short-term follow-up with a reduction of end-systolic volume of 19 % versus 6 % in those receiving coronary surgery alone. But this reduction in left ventricle volume was small compared with the previous observational studies in which volume reductions of 33 %27 and 72 %25 were achieved. There was no significant difference between the two treatments for the primary outcome at 4-year follow-up. There were also no differences between the two groups in terms of secondary outcomes, including repeat admission to hospital, symptoms or quality of life.28 SVR added to coronary bypass surgery does not appear to improve quality of life compared with coronary bypass surgery alone, but does increase healthcare costs.29
There has been a great deal of discussion aimed at reconciling the difference between the observational data supporting SVR and the findings from the STICH trial. A secondary analysis examined the influence of baseline left ventricular volumes and LVEF on outcomes. Contrary to the widely held view by surgeons enthusiastic about SVR that patients with larger, already remodelled left ventricles would benefit from coronary bypass surgery plus SVR instead of isolated coronary surgery, patients with smaller baseline left ventricular end-systolic and end-diastolic diameters were more likely to benefit, suggesting a role for SVR before extensive remodelling has occurred.30,31 Furthermore, the extent of myocardial viability in the dysfunctional anterior wall does not appear to be an important determinant of survival in patients undergoing SVR.32
It is difficult to imagine another trial of this nature, which was an enormous international effort, being carried out. The vital questions for surgeons willing to take on these patients are: how much asynergy exists; is there sufficient compensatory muscle to restore function; what is the ventricular volume? It could be that a smaller randomised trial with a more homogeneous patient group, minimal or no angina and agreed physiological end-points could be conducted.
The Role for Mitral Valve Repair
Mitral regurgitation as a result of left ventricular dysfunction and remodelling is a common feature in patients with coronary artery disease and HFrEF, especially after an inferior or infero- lateral myocardial infarct. Ischaemic mitral regurgitation is primarily, but not exclusively, a ventricular disease arising from mitral annular dilatation and restricted closure of the mitral valve leaflets due to tethering associated with left ventricular wall dysfunction.33
Ischaemic mitral regurgitation is a powerful marker of poor prognosis in patients with coronary artery disease and left ventricular dysfunction.34,35 The results of surgery for ischaemic mitral regurgitation are unproven and unpredictable in improving patient outcomes and have not been tested against medical treatment in a prospective randomised trial. Unfortunately, surgical mitral valve repair is often not durable in ischaemic mitral regurgitation due to progression of the underlying left ventricular dysfunction.36 Data from the (Randomised Ischemic Mitral Regurgitation (RIME) trial, a prospective randomised comparison of coronary bypass surgery alone versus surgery combined with mitral annuloplasty for moderate ischaemic mitral regurgitation, showed a significant improvement in the primary outcome, myocardial oxygenation consumption at 1 year (22 % increase from baseline for coronary bypass gratfing plus mitral annuloplasty versus 5 % for coronary bypass gratfing alone; p<0.001).37 There was also greater symptomatic improvement and significant reverse remodelling. This confirmed and extended the findings of a previous randomised trial of moderate ischaemia.38 Data from the National Heart, Lung, and Blood Institute Cardiothoracic Surgery Network randomised trial for severe ischaemic mitral regurgitation reported greater durability with mitral valve replacement compared with repair among experienced surgeons.39 Unfortunately, myocardial viability was not examined in this study and the trial was not powered to assess clinical outcomes.
Transcutaneous mitral valve repair is of special interest in high-risk surgical patients. Promising results have been reported in such patients who remain symptomatic despite modern medical treatment and cardiac resynchronisation therapy.40 There is a randomised trial of transcatheter valve repair versus medical management currently underway that may clarify whether targeting the mitral valve in addition to medical treatment improves the outcome of patients with ischaemic mitral regurgitation.41
Conclusions
As the severity of left ventricular dysfunction increases, so does the potential benefit of surgery to the patient. Unfortunately, the clarity of indications for coronary artery bypass grafting decreases. Following the results of the STICH trial, surgical revascularisation offers improved survival and quality of life, particularly in patients with more extensive three-vessel coronary disease and the greatest degree of left ventricle systolic dysfunction and remodelling. Unfortunately, these same patients are at the greatest short-term risk of death following coronary artery bypass surgery. SVR does not appear to add to the clinical benefit of coronary surgery in patients with more severely remodelled ventricles, but the STICH trial suffered from not having the most suitable patients enrolled. Concomitant mitral valve surgery is warranted in patients undergoing coronary bypass surgery with moderate or severe ischaemic mitral regurgitation. The discussion with patients has to focus on the balance between the short-term risks of an operation versus the potential for long-term benefit.
It is critical for the management of these complex patients that they are fully assessed by a heart team in a multi-disciplinary setting, which includes heart failure cardiologists, surgeons, intensivists, nurses and physiotherapists and possibly patients who have recovered from these procedures.