Innovative patient-centred interventions are required to improve the self-management of complex, costly and relapsing conditions, such as heart failure (HF). Mobile health (mHealth) technology is a promising tool that could potentially help us reach these goals, but evidence of its clinical benefits and the security of personal health information is still scarce. In view of the increasing number of research activities involving mHealth apps and the EU’s reference framework for the digitalisation of health, this article aims to summarise helpful information for physicians and researchers when assessing the potential benefits of mHealth apps for HF patients.1–3
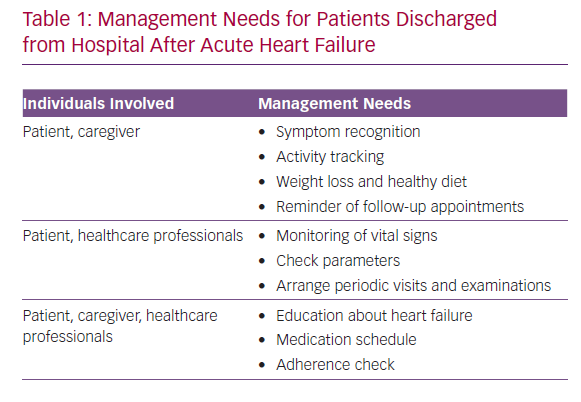
The management needs of patients discharged from hospital after new-onset acute HF and the individuals involved are shown in Table 1. The medical goals of treatment include the prevention of re-hospitalisation and major adverse cardiac events. Meaningful goals for the patient revolve around clinical stability, autonomy and recovery of function to allow the continuation of daily activities, including work and caregiving. Within this framework, technology may offer self-management systems that facilitate home monitoring, potentially increasing patient adherence and favouring the efficient integration of everyone involved in disease management.
The Rationale for Developing mHealth Apps for
HF Patients
Clinical outcomes in HF are influenced by patients’ knowledge, self-management skills and readiness to seek early care for symptoms.4 Polypharmacy, defined as the use of at least five medications, is almost the rule in HF and makes adherence to treatment plans complex. Moreover, extracardiac multimorbidity is common among HF patients and significantly contributes to polypharmacy, disability and worse outcomes.5 HF places a burden on daily routines. Patients, particularly those in the more severe stages of HF, need to frequently monitor physiological parameters and may find the pressure to adhere to prescribed medication, diet, physical activity and follow-up plans stressful.
The WHO defines mHealth as the “medical and public health practice supported by mobile devices, such as mobile phones, patient monitoring devices, personal digital assistants, and other wireless devices.”6 As of January 2019, there were 5.1 billion smartphone users worldwide, equating to 67% of the population in Europe and 78% in the US, and demonstrating a 4.2% growth rate in mobile connectivity compared to 2018.7 Personal mobile devices may soon be available to most HF patients or their caregivers.
The use of mHealth apps can improve education on different aspects of HF and promote the importance of self-care. Such apps can enable the patient to self-monitor symptoms and vital signs in order to actively participate in the control and management of the disease. Patients can record physical activity and daily moods, and profit from notification systems that help them keep track of medication consumption and follow the prescribed treatment plan. Cameras in mobile phones may also be used to record medical documents; for example, ECGs. Hence, mHealth apps have great potential to facilitate HF management.
The Digital HF Patient: Meeting the Challenges
Various reviews have previously assessed mHealth apps for HF. Some analyse apps that are not available on the market but are prototypes or have been used for feasibility purposes.9–14 Others are not based on systematic and well-recognised classification methods and many include tools that are more generally related to tracking symptoms or medication consumption. Masterson Creber et al. conducted a systematic assessment of 34 mHealth apps that were commercially available as of January 2016 using the Mobile Application Rating Scale (MARS), an accurate and complex analytical tool; only one of these apps, Heart Failure Health Storylines, had been specifically developed for HF.14,15
HF is a disease of the elderly: >80% of prevalent cases in the US and Europe are aged >65 years, with a median age at onset of 76 years.16 Physical barriers, encompassing sensory, motor and cognitive changes related to normal ageing, and poor acceptance of the use of smartphones by the older population have been reported in the literature.17,18 The proportion of older people who own and use a smartphone decreases sharply with increasing age, from 59% in those aged 65–69 years to 31% of those aged 75–79 years, and just 17% of people aged >80 years.19 Health problems and disability are the most common reasons for not using digital technologies. Many older people lack confidence in their ability to learn about and properly use electronic devices: over three-quarters of US subjects aged >65 years reported that they needed someone to set up and show them how to use a new electronic device.19 This attitude may undermine patients’ motivation to engage with mHealth apps, limit their perceived value and lead patients to poorly rate their ease of use.
Physical barriers and poor self-confidence may be impossible to overcome when users are faced with poorly designed technology. mHealth apps dedicated to HF self-care should take into account the difficulties a patient is likely to experience and give specific consideration to user-centric design features.17 Issues that have been highlighted as particularly important in the literature include easy navigation, restricting the number of items in the menu, streamlining the data entry processes, making recovery from errors clear and simplifying visualisations of data patterns.
Analysis of Commercially Available mHealth Apps
for HF Self-care
Our review focused on apps to improve HF self-care that were available to download via Google Play and Apple’s App Store. The keywords ‘heart failure’, ‘cardiac failure’ and ‘congestive heart failure’ were used to search for apps. This search was carried out in all European languages, but not in Arabic, Russian, Japanese or Chinese due to a language barrier.
The original search yielded more than 100 apps for heart diseases. We excluded apps designed for physicians; those relating to general cardiovascular conditions, such as hypertension or AF; and those managing medications in general or physical activity only. After the exclusion criteria were applied, 10 apps were downloaded.
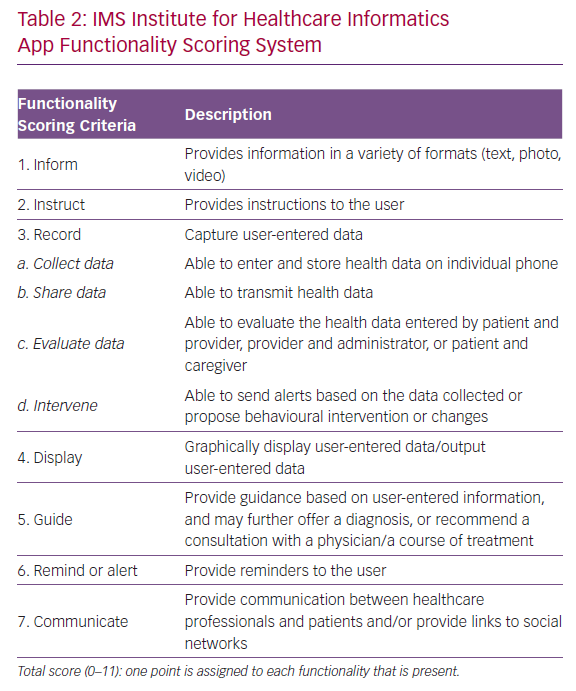
The strengths and weaknesses of the selected apps as innovative tools for HF management were independently evaluated using the 11-item IMS Institute for Healthcare Informatics app functionality scoring system (Table 2).8 The higher the score, the more complete and potentially helpful the app is. Unlike other assessment scores, including the MARS, the IMS scoring system is based on an independent and objective assessment of app functionality and does not reflect subjective patient/user or physician evaluation or evidence that users benefit from app use from an outcomes perspective.15
Results
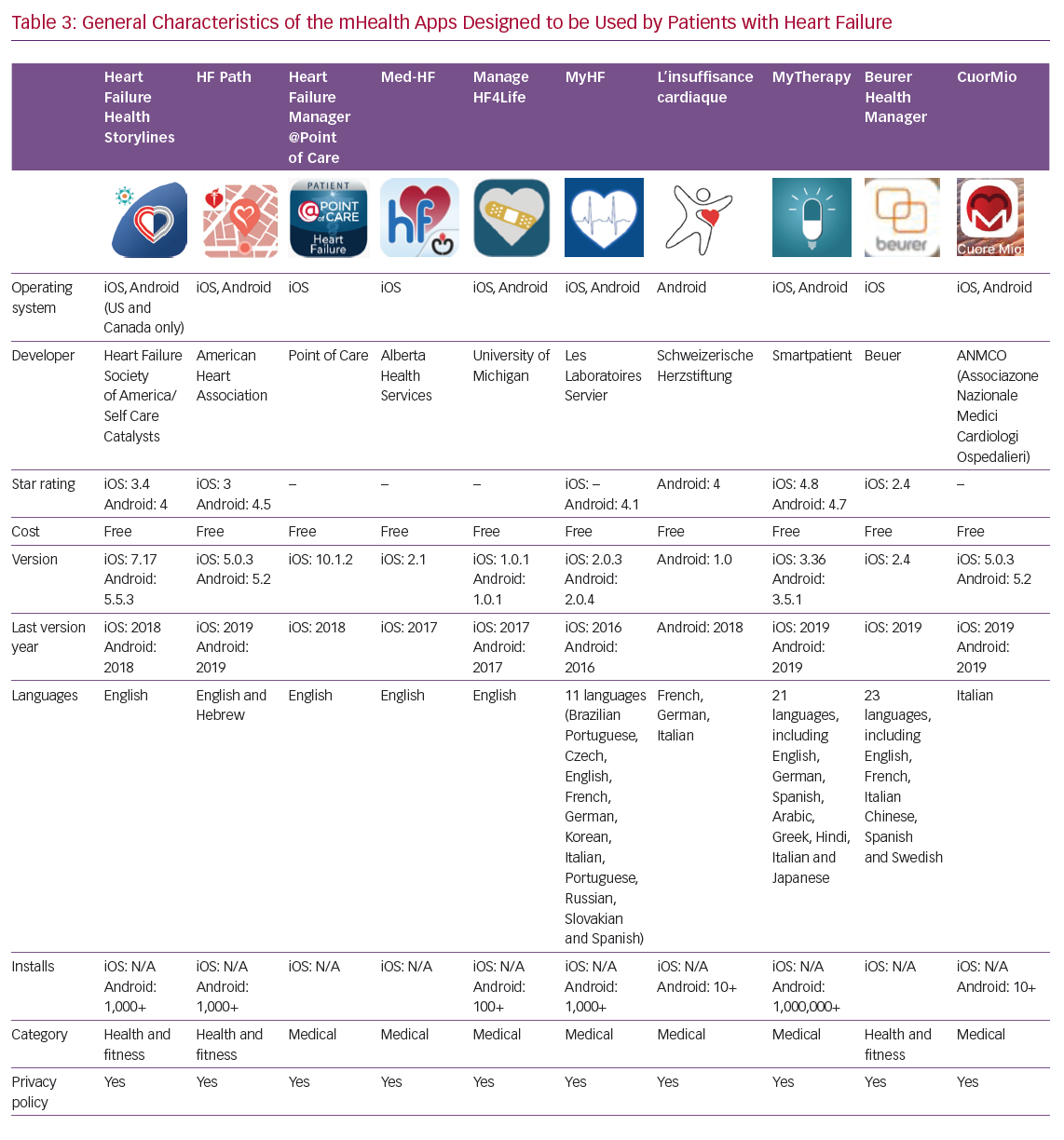
Details of the 10 apps analysed are given in Table 3. Four apps have been developed by scientific societies: HF Path (American Heart Association), Heart Failure Health Storylines (Heart Failure Society of America), CuorMio (Italian Association of Hospital Cardiologists) and Living with Heart Failure (Swiss Federation of Cardiology); the others by private companies. All apps have been developed for Android and iOS. Seven of the apps were developed in the US and three in Europe. Four of them may be considered multilingual; however, the majority are only available in English. All apps are free and most require registration before they can be used.
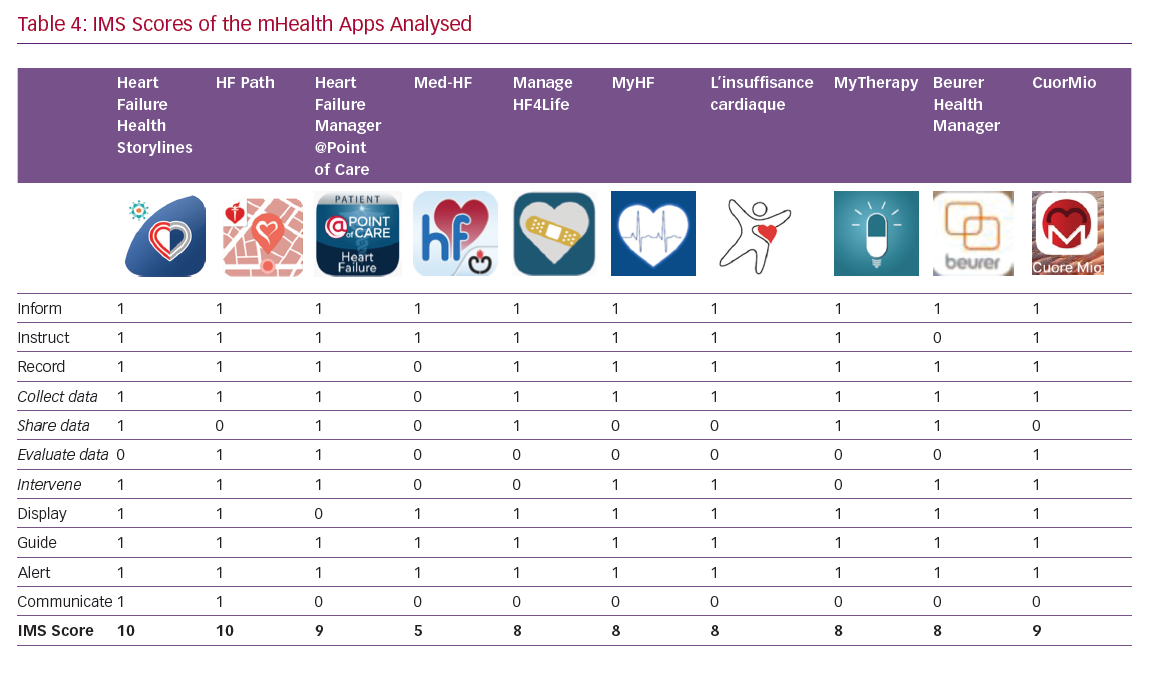
The itemised and total IMS scores for the apps are given in Table 4. The majority of the apps performed well, with nine out of 10 IMS items achieving a score >8. These items are discussed in more detail below.
Information
All apps include large educational sections on the disease and about the purpose of the app. They have areas devoted to explaining the importance of the different HF self-care domains (symptoms and signs of HF, physical activity, medication regimens, dietary intake, daily moods, education and use of sensors).
Instruction
User instructions are included in all of the apps, but provide different levels of detail. Instructions may therefore be difficult for the beginner to comprehend and training is required.
Record and Display
The apps have mainly been designed for HF self-management and to prompt users to seek early care for symptoms. They all track vital parameters (mainly blood pressure, heart rate and weight) and display them graphically. All record medication plans and send users reminders to take medication. Data are usually displayed using a colour-coding scheme and use a weekly calendar format. Data relating to vital signs are displayed in a line graph, so daily fluctuations are easy to see. The mHealth apps rarely deal with daily mood and dietary intake. Facilities to record exercise and activity, which are included in many smartphones, are not often integrated in the apps.
‘Share data’ and ‘evaluate data’ are the most critical sections; clinical data entered into the smartphone may be evaluated by the patient, caregiver and healthcare professional (HCP). The regular transmission of clinical data – which may be crucial – together with possible behavioural interventions based on the data collected is currently rarely incorporated into apps (Table 4). It must be underlined that regular transmission of clinical data implies close monitoring by HCPs and requires a dedicated organisation to respond quickly to requests from app users.
Guide
The apps perform well as a guide. All monitor vital signs and symptoms and advise the user when these parameters are out of range or his/her quality of life is getting worse. In some cases, the message may be sent directly to the physician or caregiver (@POINTofCARE and MyHF), but more often the patient is advised to contact his/her doctor.
Reminder or Alert
Reminder functions that enhance adherence to medication are included in all of the apps. Patients’ smartphones issue an alert, reminding app users that they are due to take their medication; this alert can be text only or text and an alarm. CuorMio requires the user to switch off the alarm, confirming that their medication has been taken.
Communication
This is important, since the functionality and effectiveness of the app may be improved through HF patients’ participation in social media and virtual/online support groups. Two apps, Heart Failure Health Storylines and HF Path, have a section detailing how users can connect with other HF patients through the group chat function or through the American Heart Association/American Stroke Association support network. These features may be relevant in addressing users’ fear of losing face-to-face contact, which is often quoted as a barrier to technology use, particularly by older people.
Several other aspects of the apps were also evaluated. The extra-cardiac comorbidities that often accompany HF, such as diabetes, chronic obstructive pulmonary disease, renal failure and anaemia, are not always considered. Two apps (@POINTofCARE and MyHF) include comorbid diseases as a list, but do not have features that monitor them over time. Heart Failure Health Storylines and CuorMio allow users to check comorbidities through blood test profiling. However, in general, the attention paid to other important diseases seems modest for all of the apps.
During the development of its HealthManager app and platform, Beuer paid particular attention to the protection of health data collected and saved during user registration and has obtained a security certificate. In many other mHealth apps, this important legal item has not been clarified.
Finally, smartphones have cameras that may play an important role in a novel concept of HF management. The CuorMio app, among other functions, can store files relating to the patient’s treatment. These files can be added by all HCPs treating the patient, thus building a portable electronic health record that moves with the patient.
Future Development Suggestions
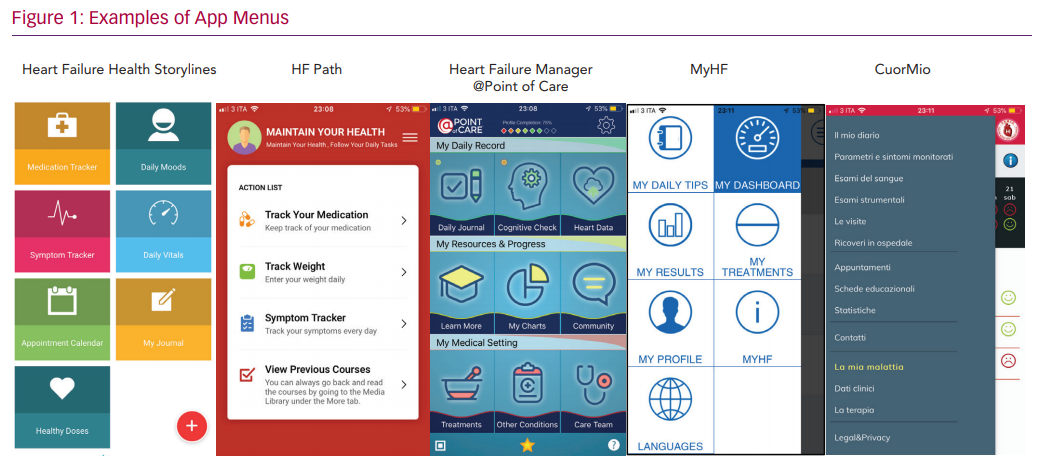
The mHealth apps we analysed have been designed for HF patients and appear to be simple and easy to navigate. However, a hierarchical approach with different menus is used by all and may require a fair amount of technological skill to navigate (see examples in Figure 1). Since older people may need assistance to use many smartphone features, instruction and support is critical. Family members are an invaluable source of help for many older patients, but not all caregivers – who are often spouses and of an older age themselves – may be able to overcome the digital challenges. However, when older patients and caregivers are provided with guidance/trained to use mHealth apps, as shown by several usability analyses, feedback is positive and participants may follow mHealth app instructions, even over long periods of time.20,21
Interdisciplinary approaches to mHealth app design and development are paramount. Technology experts, HCPs and end-users (both patients and their caregivers) need to be involved. Patients should be consulted in the design and preferably act as co-developers of the tools. A wide range of groups needs to be included in assessment studies and it is important to engage with patients who may be less independent and more likely experience barriers to use.
Apps as Medical Devices: A Continuing Debate
The new European Medical Devices Regulation was published in 2017 and will enter into force on 25 May 2020.22 The market access framework for EU Member States will change significantly and will probably differ from US regulation. All medical devices require CE marking, that is, the manufacturer’s claim that a product meets the essential requirements of all relevant European Medical Device Directives, that it is fit for purpose and is safe. Stand-alone software, such as an mHealth app, is currently classified based on purpose.23,24 If the app has a medical purpose – a definition that encompasses prevention, diagnosis, monitoring and the treatment of any disease – it should be considered a medical device. An app that provides patient education, monitors fitness/health/wellbeing or stores and transmits medical data without changing its purpose is not defined as a medical device.
The mHealth apps analysed in this article may be considered portable systems for improving knowledge about HF self-management, recognising and seeking early care for symptoms and increasing adherence to treatment plans. The monitoring of physiological parameters, such as blood pressure, heart rate, weight and oxygen saturation, may be considered an important part of HF self-care: data input on the app is simply a patient note when the parameters are not obtained through other devices. Hence, the mHealth app is a sort of data repository that can only be used by the patient. HCPs will consult the mHealth app only during regular patient visits or when specific alerts occur that suggest patients consult them.
The border is blurred by the transmission of data. mHealth apps that regularly transmit data to hospitals or HCPs, as now happens with implantable devices, can result in the HCP taking an action – be it changing a patient’s therapy, calling them to arrange an appointment or referring them for an urgent consultation. Such mHealth apps would be considered medical devices.
The debate is still ongoing. EU Member States will probably deliberate as to whether or not individual mHealth apps are devices based on the new Medical Devices Regulation. However, it is important to note that the presence of a CE mark does not imply that the app meets best practice or has been tested for accuracy or clinical benefits.
What Level of Evidence is Needed to Assess the
Value of mHealth Apps?
The IMS Institute for Healthcare Informatics highlighted the generation of evidence of value – in terms of behaviour change and improved health outcomes from using apps – as one of the most important areas for future research in mHealth. Lack of efficacy testing is one of the biggest barriers to adopting mHealth apps. Most reports on the evaluation of mHealth apps are small pilots and descriptive studies. The randomised controlled trial (RCT) paradigm, the cornerstone of evidence-based medicine, has been applied not only to drugs and devices but also to educational programmes and disease management strategies. However, the cost and complexities of running RCTs may render the clinical trial model inappropriate or impractical for the validation of mHealth apps for self-care: the technology evolves very rapidly and this tumultuous pace may be at variance with the times needed to plan, approve and execute an RCT.
The review by Cajita et al. is exemplary in this sense: among nine studies that assessed the efficacy of mHealth-based intervention on HF outcomes performed in the past decade, eight used telemonitoring systems of health status, but the wide range of technologies (including invasive devices), provider response, duration and health outcomes assessed made meta-analysis impossible.10 The studies reported contradictory findings relating to mortality, morbidity, functional status, self-rated quality of life and self-care. Patient adherence rates to mHealth intervention were consistently low: only one study achieved 100% adherence; 20% of the intervention group in another study failed to even start due to difficulties operating their smartphone’s browser; two other studies reported attrition rates of 60% and 30%, respectively, due to technical difficulties. This review highlights the critical issue with mHealth: high dropout rates due to poor function or problems with acceptability.
An app is basically a digital way to access information and functions. It is a conduit to appropriately designed content based on the foundations of HF self-care. The role of scientific societies is pivotal in setting agreed content standards. The European Society of Cardiology, American Heart Association and American College of Cardiology, and Heart Failure Society of America have produced documents on the core aspects of self-care and recommendations for self-care education programmes are incorporated into international HF guidelines.25–27 Close adherence to those principles should be a mandatory requirement for any mHealth app focused on HF patients.
Alternative strategies for efficient and accurate validation should take into account the evolving nature of apps and the requirement for continuous refinement to ensure tools are sufficiently attuned to patients’ need for sustained use. Previous studies have shown that daily use declines consistently after the first month.28
Close cooperation between scientific societies and information technology experts is needed to foster the appropriate development and continuous upgrade of mHealth apps and to set standards for validation. Studies should be planned to assess the consistent functioning, usability and acceptability of the app and to verify its sustained uptake at meaningful time intervals to measure its impact on outcomes, such as adherence or quality of life. Sex-balanced cohorts should be enrolled, including individuals of different ages and levels of digital and health literacy. Unstructured user experience ratings are important at the pilot testing stage to refine the app. Validated scales such the IMS or the Mobile App Rating Scale should be used to test fully developed versions.8,15 Initiatives, such as the NHS Apps Library in the UK, exemplify useful assessment frameworks for apps based on a set of criteria, including legislative and regulatory issues, standard or best practice and national health policies.29
Conclusion
There is great potential for mHealth apps to foster patient engagement in HF self-care and improve interaction with HCPs in a cost-effective way. To ensure that the tools add value and really meet patient needs, patients need to participate in their design, development and refinement.
Scientific societies will play a pivotal role in the development of guidance on the use of mHealth apps in clinical practice, suggesting when (e.g. after discharge, as an outpatient), to which type of patient (e.g. based on literacy level, caregiver support) and under which terms of use it is advisable to prescribe an mHealth app for HF self-care. Scientific societies also have a role in reassuring HCPs and patient organisations about the quality and effectiveness of the approach.
The next target for digital HF self-care should be the integration of multimorbidity monitoring and management. This development should reflect multidisciplinary cooperation as fostered by professional scientific societies.