Dear Editor,
The use of extracorporeal membrane oxygenation (ECMO) as salvage therapy in the most severe cases of acute respiratory distress syndrome (ARDS) has been associated with reduced mortality, particularly at high-volume centres. We report a case series of seven patients with coronavirus disease 2019 (COVID-19)-associated ARDS treated with ECMO.
In select COVID-19 patients suffering from severe ARDS refractory to conventional therapy, ECMO might be an outcome altering therapy. Respiratory ECMO Survival Prediction (RESP) score appears to be a reliable prognostication tool in selecting COVID-19 patients most likely to benefit from ECMO. Early and frequent evaluation of critically ill COVID-19 patients for ECMO therapy could facilitate timely initiation, and ultimately, favourable outcomes. ECMO is a finite resource, and thus must be used judiciously, especially in the midst of a pandemic where all resources are stretched thin.
ECMO is a well-established salvage therapy in treatment of severe refractory ARDS. Venous–venous ECMO (VV-ECMO) is a modified cardiopulmonary bypass system in which venous blood is removed from the body and circulated through an artificial membrane lung and has successfully been deployed in the treatment of patients with severe ARDS. The initiation of VV-ECMO allows for ultra-lung protective/‘lung rest’ ventilation in ARDS patients with poor lung compliance. During the H1N1 influenza pandemic, a meta-analysis of 266 patients with severe ARDS supported with VV-ECMO showed a survival rate of 72.5%, albeit with prolonged hospitalisations.1
While previous reports on VV-ECMO in ARDS are encouraging, initial reports of its use in Chinese COVID-19 patients have been less promising. Of the six patients placed on ECMO in Wuhan, China, only one survived to hospital discharge.2 In Shanghai, only four of eight patients survived to ECMO decannulation.3 Early US data are similarly grim. A compiled study of 32 patients from nine different centres in the US showed a mortality rate of 31%, with 53% patients still on ECMO after 3 weeks.4 However, there remains a paucity of literature on its utilisation and efficacy in the treatment of COVID-19-associated ARDS, especially among US patients.
Methods
Baylor-St Luke’s Medical Center is a large, academic quaternary hospital with 661 beds in the Texas Medical Center, Houston, TX, US. It serves as a centre for advanced heart failure and heart transplantation, with a robust volume of mechanical circulatory support, including ECMO (approximately 100–150 per year). We present a case series of seven consecutive polymerase chain reaction-confirmed diagnoses of COVID-19 patients admitted to our centre between 29 March and 8 May 2020. Prior to the initiation of ECMO, patients were screened for major comorbidities, with an absolute age cut-off age of >65 years and predicted survival based on a RESP score of <40%.
Results
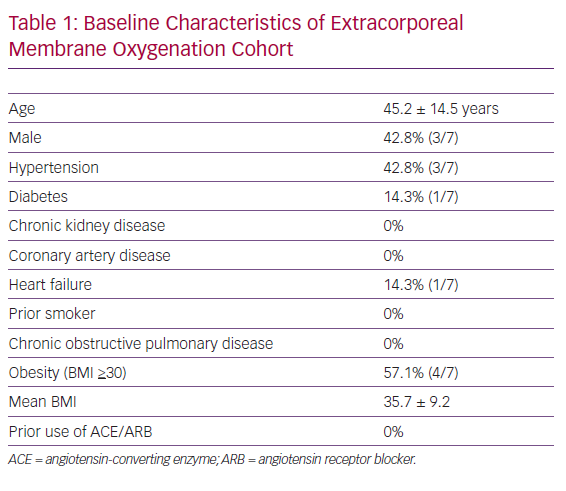
The mean age of our cohort was 45 years and comprised three men and four women. The most common baseline comorbidities included obesity (four patients, mean BMI: 35.7) and hypertension (three patients). There was no history of smoking, chronic obstructive pulmonary disease, asthma, chronic kidney disease or coronary artery disease. Only one of seven (14%) patients had a prior history of diabetes mellitus, heart failure or angiotensin-converting enzyme/angiotensin receptor blocker use. A comprehensive list of baseline characteristics is provided in Table 1.
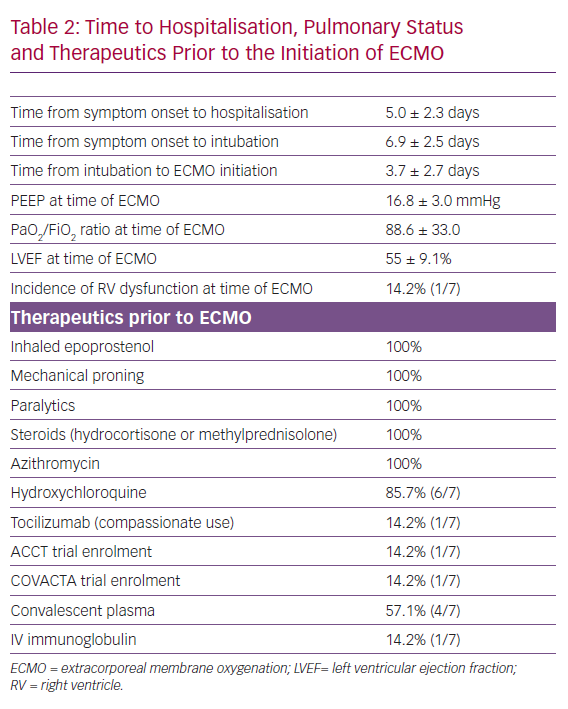
Patients presented to the hospital on average 7 days after onset of symptoms, spent 1.9 days in hospital prior to intubation and 3.7 days from the time of intubation to the initiation of ECMO. All had refractory hypoxia, despite lung-protective ventilation; neuromuscular blockade; inhaled epoprostenol; and underwent prone position ventilation. Average positive end-expiratory pressure was 16.9 mmHg, with tidal volume of 5.75 ml/kg of ideal body weight and PaO2/FiO2 ratio of 84.5 prior to ECMO initiation. There was evidence of left and right ventricle dysfunction based on gross visual assessment in one patient, with a mean left ventricular ejection fraction of 55 ± 9%. The average RESP score was 3.7 at the time of ECMO cannulation. Hydroxychloroquine (six patients), azithromycin (seven patients) and hydrocortisone (100%) use was almost universal in our study cohort. Four patients were treated with at least one dose of convalescent plasma, while tocilizumab (for compassionate use 1/7, 14%) and IV immunoglobulin (1/7, 14%) were rarely used (Table 2). One patient was enrolled in the Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia (COVACTA), and another patient was enrolled in the Adaptive COVID-19 Treatment Trial (ACTT) for remdesivir.
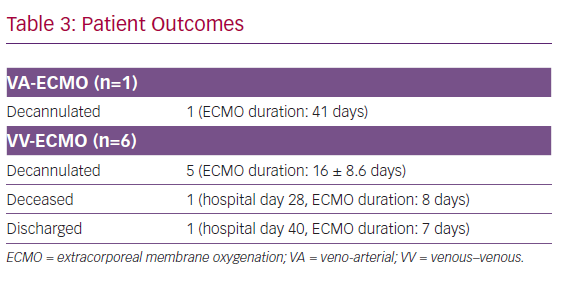
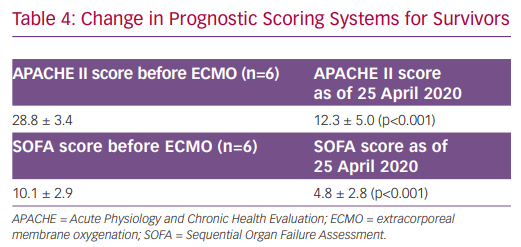
At time of this report, one patient died from haemorrhagic shock on hospital day 23 after 8 days of VV-ECMO. The remaining six patients were decannulated from ECMO, and one patient was discharged home on hospital day 40 (Table 3). For all surviving patients, Acute Physiology and Chronic Health Evaluation (APACHE) II scores decreased from 28.8 at the time of ECMO initiation to 11.8 (p<0.001) over their clinical course. Similarly, Sequential Organ Failure Assessment (SOFA) scores dropped from 10.1 to 4.4 (p<0.001) (Table 4).
Discussion
ECMO is a well-established salvage therapy in the treatment of severe refractory ARDS. However, its role in the treatment of COVID-19-associated ARDS currently remains unknown. Our report describes the clinical course of COVID-19 patients treated with ECMO at a major high-volume academic medical centre in the US. The key findings of our study are as follows. First, the majority of our patients were successfully weaned off ECMO and continue to show clinical improvement. Second, COVID-19 patients require a prolonged runtime on ECMO prior to being weaned off. Third, the RESP score appears to be a reliable measure in predicting outcomes among COVID-19 patients treated with ECMO.
Although clinical guidelines for the management of COVID-19 have been published by the WHO and Surviving Sepsis Campaign, the role of ECMO as salvage therapy for severe ARDS remains unclear, in part due to the lack of published evidence.5,6 Current observational studies from China and the US have not been encouraging.2,3 Our retrospective analysis provides evidence to the contrary. There are several plausible explanations for our findings. We are very proactive in ensuring early (48–72 hours) evaluation of our severe ARDS COVID-19 patients for ECMO cannulation if they show no improvement or clinical worsening on conventional therapy. As a matter of institutional practice, ECMO eligibility is discussed on daily bedside rounds for each of our COVID-19 intensive care unit (ICU) patients. As previously mentioned, ECMO renders clinical benefit by allowing ‘lung rest’ ventilation, and thus minimising the risk of ventilator-induced lung injury in non-complaint lungs. Therefore, it is imperative that ECMO be initiated early on in the disease process before irreversible lung damage ensues. The average number of days on mechanical ventilation prior to ECMO in the Shanghai cohort was just over 10 days, whereas that of our cohort was significantly lower at 3.7 days.3
The optimal selection of patients most likely to benefit from ECMO also appears to have contributed to the differences in outcomes between our cohort and the Shanghai cohort. In our study, we used the RESP score to calculate the probability of hospital survival and used 40% as our arbitrary cut-off for who was offered treatment with ECMO. On the contrary, ECMO was offered more broadly in the Shanghai cohort to any patient that met any of the following criteria, despite optimal mechanical ventilation: PaO2/FiO2 <50 mmHg for >1 hour; PaO2/FiO2 <80 mmHg for >2 hours; and the existence of uncompensated respiratory acidosis with PH <7.2 for >1 hour. It is crucial to appreciate that ECMO is a resource-intensive, highly-specialised and expensive form of life support, with the potential of significant complications, and thus should only be reserved for truly refractory cases that are most likely to benefit from it. While the RESP score has not been directly validated in the COVID-19 cohort, our study suggests that it still remains a relevant and reliable predictor of outcomes.7
Another key finding of our study was the prolonged ECMO runtime among our COVID-19 patients. Anecdotal reports and our experience in the COVID-19 ICU suggest that COVID-19 patients require supportive care for a much longer time than conventional ARDS patients. Houston has not seen the same surge of COVID-19 patients as other global epicentres, such as Wuhan, Lombardy and New York, and this has allowed us to allocate our ECMO circuits to our COVID-19 patients for extended durations. This too has contributed to our favourable outcomes. However, it is important to note that ECMO is a finite resource, and thus must be used judiciously, especially in the midst of a pandemic where all resources are stretched thin.
Conclusion
ECMO can be a valuable tool in the management of COVID-19-associated ARDS if implemented early and in carefully selected patients.